

Torsemed

Ask a doctor about a prescription for Torsemed

How to use Torsemed
Package Leaflet: Information for the Patient
Warning! Keep the Leaflet! Information on the Immediate Packaging in a Foreign Language.
Torsemed(Torasemid - 1 A Pharma)
20 mg, Tablets
Torasemidum
Torsemed and Torasemid - 1 A Pharma are different trade names for the same drug.
Read the Leaflet Carefully Before Taking the Medication, as it Contains Important Information for the Patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you by a doctor. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet:
- 1. What is Torsemed and What is it Used For
- 2. Important Information Before Taking Torsemed
- 3. How to Take Torsemed
- 4. Possible Side Effects
- 5. How to Store Torsemed
- 6. Contents of the Packaging and Other Information
1. What is Torsemed and What is it Used For
Torsemed is used to treat:
- Edema caused by excessive fluid in the body.
Torsemed is a diuretic, which means it increases urine production.
2. Important Information Before Taking Torsemed
When Not to Take Torsemed
- allergyto:
- torasemideor any of the other ingredients of this medicine (listed in section 6)
- similar medicines called "sulfonylurea derivatives" used to treat high blood sugar, containing active substances with names ending in "-mid"
- renal failurewith inadequate urine production
- severe liver dysfunctionwith loss of consciousness
- low blood pressure
- reduced blood volume
- breastfeeding
- low potassium or sodium levels in the blood
- severe urinary tract disorders, e.g. due to prostate enlargement
- gout
- irregular heart rhythm
- taking medications used to treat infections, such as cefixime, cefuroxime, cefaclor, cefalexin, cefadroxil, cefpodoxime proxetil, kanamycin, neomycin, gentamicin, amikacin, or tobramycin
- renal dysfunctioncaused by medications that damage the kidneys.
Warnings and Precautions
If any of the following situations apply to you, discuss them with your doctor before starting Torsemed:
- acid-base balance disorders
- blood morphology disorders
- diabetes
- lithium use, a medication used to treat depression.
If you are taking Torsemed continuously, your doctor will regularly order blood tests, especially if you are taking other medications, have diabetes, or have an irregular heart rhythm.
Children and Adolescents
Torsemed is not recommended for children and adolescents under 18 years of age.
Torsemed and Other Medications
Tell your doctor or pharmacistabout all medications you are taking, have recently taken, or plan to take.
The following medications may affect Torsemed and vice versa:
- medications used to treat high blood pressure, especially those containing active substances with names ending in "-pril"
- medications that increase the heart's ability to pump blood, such as digitoxin, digoxin, or methyldigoxin
- medications used to treat diabetes
- probenecid(a medication used to treat gout)
- medications used to treat inflammation and pain, such as acetylsalicylic acid or indomethacin
- sulfasalazine, mesalazine, or olsalazine(medications used to treat chronic inflammatory bowel diseases)
- medications used to treat infections, such as cefixime, cefuroxime, cefaclor, cefalexin, cefadroxil, cefpodoxime proxetil, kanamycin, neomycin, gentamicin, amikacin, or tobramycin
- platinum compounds, such as cisplatin (a medication used to treat cancer)
- lithium(a medication used to treat depression)
- theophylline(a medication used to treat asthma)
- certain muscle relaxantswith names ending in "-curonium" or "-curium"
- all medications used to treat constipation
- medications containingcorticosteroids, such as hydrocortisone, prednisone, or prednisolone
- cholestyramine(a medication used to reduce blood fat levels)
- adrenalineor noradrenaline(medications used to increase blood pressure or accelerate heart rate)
- warfarin, a medication used to treat blood clots.
Pregnancy and Breastfeeding
If you are pregnant, breastfeeding, think you may be pregnant, or plan to become pregnant, ask your doctor or pharmacist for advice before taking this medication.
- PregnancyTorsemed should only be used during pregnancy if your doctor considers it absolutely necessary. In this case, use the lowest possible dose.
- BreastfeedingTorsemed should not be usedin breastfeeding women, as it may harm the baby.
Driving and Operating Machinery
While taking Torsemed, you may experience dizziness or drowsiness, especially at the beginning of treatment, when increasing the dose, changing the medication, starting to take an additional medication, or consuming alcohol. Do not drive or operate machinery if your concentration is impaired.
Torsemed Contains Lactose Monohydrate
The medication contains sugar lactose. If you have been diagnosed with intolerance to some sugars, you should consult your doctor before taking this medication.
3. How to Take Torsemed
Always take this medication exactly as your doctor has told you. If you are unsure, ask your doctor or pharmacist.
Recommended doseis:
Treatment of edema caused by excess fluid in the body
- 1 tablet (20 mg) once a dayIf necessary, your doctor may gradually increase the dose to 1 tablet (20 mg) once a day.
Dividing the Tablet
The tablet can be divided into equal doses.
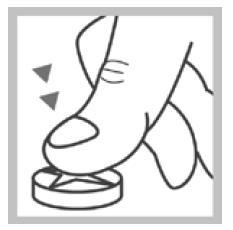
Place the tablet on a hard, flat surface, with the dividing groove facing up. Press your finger on the center of the tablet and divide it into four equal parts.
Use in Children and Adolescents
Due to insufficient experience with torasemide in children and adolescents, Torsemed is not recommended for patients under 18 years of age.
Liver Dysfunction (Except in Severe Cases)
Treatment requires caution due to the possibility of increased torasemide levels in the blood.
Method of Administration
Take the tablets every morning, regardless of meals, without chewing, with 100 ml of water (about half a glass).
Duration of Treatment
The duration of treatment is determined by your doctor. Torsemed can be used continuously for several years or until the edema (excess fluid in the tissues) subsides.
Taking More Than the Recommended Dose of Torsemed
Seek medical attention immediately.
Overdose may cause excessive urine production, drowsiness, confusion, weakness, dizziness, low blood pressure, circulatory collapse, or stomach upset.
Take the remaining tablets and the packaging with you to help identify the medication taken.
Missing a Dose of Torsemed
Take the missed dose as soon as possible on the same day or take the next dose at the usual time the next day. Do not take a double dose to make up for the missed dose.
Stopping Torsemed Treatment
Do not stop taking Torsemed without your doctor's consent, as this may cause harmful effects and reduce the effectiveness of the treatment.
If you have any further questions about taking this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, Torsemed can cause side effects, although not everybody gets them.
Side Effects May Occur with the Following Frequency:
Common(may affect up to 1 in 10 people)
- disorders of water and mineral balance in the body, especially with restricted salt intake
- increased alkalinity in the body
- muscle cramps
- increased levels of uric acid, sugar, and fats in the blood
- decreased levels of potassium and sodium in the blood
- decreased blood volume
- gastrointestinal disorders, such as loss of appetite, stomach pain, nausea, vomiting, diarrhea, or constipation
- increased activity of certain liver enzymes, such as gamma-GT
- headaches
- dizziness
- fatigue
- weakness
Uncommon(may affect up to 1 in 100 people)
- increased levels of urea and creatinine in the blood
- dry mouth
- tingling or numbness in the hands and feet
- difficulty urinating (e.g., due to prostate enlargement)
Rare(may affect up to 1 in 10,000 people)
- vasoconstriction due to blood thickening
- lower than normal blood pressure
- circulatory disorders, especially when standing up
- irregular heart rhythm
- angina pectoris - a condition characterized by severe chest pain
- myocardial infarction
- fainting
- pancreatitis
- allergic reactions with itching and rash
- increased skin sensitivity to light
- severe skin reactions
- decreased red and white blood cell and platelet count
- vision disorders
- ringing or buzzing in the ears
- hearing loss
Frequency Not Known: frequency cannot be estimated from the available data
- insufficient blood flow to the brain
- disorientation (confusion).
Reporting Side Effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medication.
5. How to Store Torsemed
- Keep the medication out of sight and reach of children.
- Do not use this medication after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
- Do not store above 25°C.
- Translation of some information on the immediate packaging: Ch.-B. verwendbar bis siehe Prägung- Batch Number/Expiry Date: see imprint.
- Medications should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medications that are no longer needed. This will help protect the environment.
6. Contents of the Packaging and Other Information
What Torsemed Contains
- The active substanceis torasemide. Each tablet contains 20 mg of torasemide.
- Other ingredients are: microcrystalline cellulose, lactose monohydrate, magnesium stearate, corn starch, colloidal anhydrous silica.
What Torsemed Looks Like and Contents of the Packaging
White or almost white, round tablets with a dividing groove.
Pack sizes: 30, 60, and 90 tablets.
For more detailed information, contact the marketing authorization holder or parallel importer.
Marketing Authorization Holder in Germany, the Country of Export:
1 A Pharma GmbH
Industriestraße 18
83607 Holzkirchen
Germany
Manufacturer:
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
39179 Barleben
Germany
Lek S.A.
ul. Podlipie 16
95-010 Stryków
Poland
Parallel Importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing Authorization Number in Germany, the Country of Export:58031.03.00
Parallel Import Authorization Number:160/20
Date of Leaflet Approval: 25.06.2025
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)1 A Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TorsemedDosage form: Tablets, 5 mgActive substance: torasemideManufacturer: Polfarmex S.A.Prescription requiredDosage form: Tablets, 10 mgActive substance: torasemideManufacturer: Polfarmex S.A.Prescription requiredDosage form: Tablets, 20 mgActive substance: torasemideManufacturer: Polfarmex S.A.Prescription required
Alternatives to Torsemed in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Torsemed in Spain
Alternative to Torsemed in Ukraine
Online doctors for Torsemed
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Torsemed – subject to medical assessment and local rules.










