

Torsemed

Ask a doctor about a prescription for Torsemed

How to use Torsemed
LEAFLET INCLUDED IN THE PACKAGE: INFORMATION FOR THE PATIENT
Warning! Keep the leaflet, the information on the immediate packaging is in a foreign language!
Torsemed(Torasemid HEXAL 20 mg Tablets),
20 mg, tablets
Torasemidum
Torsemed and Torasemid HEXAL 20 mg Tablets are different trade names for the same medicine.
You should carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
You should keep this leaflet so that you can read it again if you need to.
In case of any further doubts, you should consult a doctor or pharmacist.
This medicine has been prescribed to a specific person. Do not pass it on to others. The medicine may harm another person, even if their illness symptoms are the same.
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse.
See section 4.
Table of contents of the leaflet:
- 1. What is Torsemed and what is it used for
- 2. Important information before taking Torsemed
- 3. How to take Torsemed
- 4. Possible side effects
- 5. How to store Torsemed
- 6. Contents of the package and other information
1. What is Torsemed and what is it used for
Torsemed is used to treat:
swelling caused by excess water in the body.
Torsemed is a diuretic, a medicine that increases urine production.
2. Important information before taking Torsemed
When not to take Torsemed
In case of allergyto:
- - torasemideor any of the other ingredients of this medicine (listed in section 6)
- -similar medicines called "sulfonylurea derivatives", used to treat high blood sugar, containing active substances with names ending in "-mid" in case of kidney failure, with insufficient urine production in case of severe liver dysfunction, with loss of consciousness in case of low blood pressurein case of reduced blood volumeduring breastfeedingin case of low potassium or sodium levels in the bloodin case of severe urinary tract disorders, e.g. due to prostate enlargement in case of goutin case of irregular heart rhythm
In case of taking medicines used to treat infections, such as cefixime, cefuroxime, cefaclor, cefalexin, cefadroxil, cefpodoxime proxetil, kanamycin, neomycin, gentamicin, amikacin, or tobramycin
In case of kidney function disorderscaused by medicines that damage the kidneys.
Warnings and precautions
If any of the above situations apply to the patient, they should discuss this with their doctor before starting to take Torsemed:
acid-base balance disorders
blood morphology disorders
diabetes
use of lithium, a medicine used to treat depression.
If the patient is taking Torsemed continuously, their doctor will regularly prescribe blood tests, especially if the patient is taking other medicines, has diabetes, or has an irregular heart rhythm.
Children and adolescents
Torsemed is not recommended for children and adolescents under 18 years of age.
Torsemed and other medicines
The patient should tell their doctor or pharmacistabout all medicines they are taking or have recently taken, as well as any medicines they plan to take.
The following medicines may affect the action of Torsemed and vice versa:
medicines used to treat high blood pressure, especially those containing active substances whose names end in "-pril"
medicines that increase the heart's ability to pump blood, such as digitoxin, digoxin, or methyldigoxin
medicines used to treat diabetes
probenecid(a medicine used to treat gout)
medicines used to treat inflammation and pain, such as acetylsalicylic acid or indomethacin
sulfasalazine, mesalazine, or olsalazine(medicines used to treat chronic inflammatory bowel diseases)
medicines used to treat infections, such as cefixime, cefuroxime, cefaclor, cefalexin, cefadroxil, cefpodoxime proxetil, kanamycin, neomycin, gentamicin, amikacin, or tobramycin
platinum compounds, such as cisplatin (a medicine used to treat cancer)
lithium(a medicine used to treat depression)
theophylline(a medicine used to treat asthma)
some muscle relaxantswhose names end in "-curonium" or "-curium"
all laxatives
medicines containingcorticosteroid derivatives, such as hydrocortisone, prednisone, or prednisolone
cholestyramine(a medicine used to reduce blood fat levels)
adrenalineor noradrenaline(medicines used to increase blood pressure or accelerate heart rate)
warfarin, a medicine used to treat blood clots.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to become pregnant, they should consult their doctor or pharmacist before taking this medicine.
Pregnancy
Torsemed can be used during pregnancy only if the doctor considers it absolutely necessary. In this case, the smallest possible doseshould be used.
Breastfeeding
Torsemed should not be usedin breastfeeding women, as it may harm the baby.
Driving and operating machinery
While taking Torsemed, dizziness or drowsiness may occur, especially at the beginning of treatment, when increasing the dose, changing the medicine, starting to take an additional medicine, or consuming alcohol. In case of impaired concentration, do not drive vehicles or operate machinery.
Torsemed contains lactose monohydrate
The medicine contains sugar lactose. If the patient has previously been diagnosed with intolerance to some sugars, they should contact their doctor before taking this medicine.
3. How to take Torsemed
This medicine should always be taken as directed by the doctor. In case of doubts, consult a doctor or pharmacist.
The recommended doseis:
Treatment of swelling caused by excess water in the body
¼ tablet (5 mg) once a day
If necessary, the doctor may gradually increase the dose to 1 tablet (20 mg) once a day.
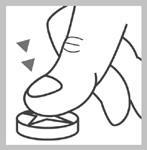
Dividing the tablet
The tablet can be divided into equal doses.
Place the tablet on a hard, flat surface, with the dividing groove facing up.
Press the finger on the center of the tablet and divide it into four equal parts.
Use in children and adolescents
Due to insufficient experience with the use of torasemide in children and adolescents, Torsemed is not recommended for patients under 18 years of age.
Due to insufficient experience with the use of torasemide in children and adolescents, Torsemed is not recommended for patients under 18 years of age.
Liver function disorders (except in severe cases)
Treatment requires caution due to the possibility of increased torasemide levels in the blood.
Method of administration
Tablets should be taken every morning, regardless of meals, without chewing, with 100 ml of water (about half a glass).
Duration of treatment
The duration of treatment is determined by the doctor. Torsemed can be used continuously for several years or until the swelling (excess fluid in the tissues) subsides.
Taking a higher dose of Torsemed than recommended
The patient should contact their doctor immediately.
Overdose may cause excessive urine production, drowsiness, confusion, weakness, dizziness, low blood pressure, circulatory collapse, or stomach upset.
The patient should take the remaining tablets and the packaging of the medicine to facilitate identification of the taken medicine.
Missing a dose of Torsemed
The patient should take the missed dose as soon as possible on the same day or take the next dose at the usual time the next day. Do not take a double dose to make up for the missed dose.
Stopping Torsemed treatment
The patient should not stop taking Torsemed without their doctor's consent, as this may cause harmful effects and reduce the effectiveness of treatment.
In case of any further doubts about taking this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Torsemed can cause side effects, although not everybody gets them.
Side effects may occur with the following frequency:
Common(may occur in less than 1 in 10 people)
disorders of water and mineral balance in the body, especially with restricted salt intake
increased alkalinity in the body
muscle cramps
increased levels of uric acid, sugar, and fats in the blood
decreased levels of potassium and sodium in the blood
decreased blood volume
gastrointestinal disorders, such as loss of appetite, stomach pain, nausea, vomiting, diarrhea, or constipation
increased activity of certain liver enzymes, such as gamma-GT
headaches
dizziness
feeling of tiredness
weakness
Uncommon(may occur in less than 1 in 100 people)
increased levels of urea and creatinine in the blood
dry mouth
tingling or numbness in hands and feet
difficulty urinating (e.g., due to prostate enlargement)
Rare(may occur in less than 1 in 10,000 people)
vasoconstriction due to blood thickening
lower than normal blood pressure
circulatory disorders, especially when standing up
irregular heart rhythm
angina pectoris - a condition characterized by severe chest pain
myocardial infarction
fainting
pancreatitis
allergic reactions with itching and rash
increased skin sensitivity to light
severe skin reactions
decreased number of red and white blood cells and platelets
vision disorders
ringing or buzzing in the ears
hearing loss
Frequency not known: frequency cannot be estimated from the available data
insufficient blood supply to the brain
disorientation (confusion).
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Torsemed
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the given month.
Do not store above 25°C.
Translation of some abbreviations on the blister pack:
Ch.-B./verwendbar bis: see imprint - Batch number/Expiry date: see embossing (on the blister pack).
Medicines should not be disposed of via wastewater or household waste containers. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the package and other information
What Torsemed contains
The active substanceof Torsemed is torasemide. Each tablet contains 20 mg of torasemide.
Other ingredients are: microcrystalline cellulose, lactose monohydrate, magnesium stearate, corn starch, anhydrous colloidal silica.
What Torsemed looks like and what the pack contains
White or almost white, round tablets with a dividing groove.
PVC/COC/PVDC/Al or Al/Al blisters in a cardboard box.
Package sizes: 30, 60, and 90 tablets.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Germany, the country of export:
Hexal AG
Industriestraße 25
83607 Holzkirchen, Germany
Manufacturer:
LEK S.A.
Podlipie 16
95-010 Stryków
Poland
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
39179 Barleben
Germany
Parallel importer:
Medezin Sp. z o.o.
ul. Zbąszyńska 3
91-342 Łódź
Repackaged by:
Medezin Sp. z o.o.
ul. Zbąszyńska 3
91-342 Łódź
CEFEA Sp. z o.o. Sp. komandytowa
ul. Działkowa 56
02-234 Warsaw
Pharma Innovations Sp. z o.o.
ul. Jagiellońska 76
03-301 Warsaw
Synoptis Industrial Sp. z o.o.
ul. Szosa Bydgoska 58
87-100 Toruń
CANPOLAND SPÓŁKA AKCYJNA
ul. Beskidzka 190
91-610 Łódź
Marketing authorization number in Germany, the country of export:58015.03.00
Parallel import authorization number: 272/22 Date of leaflet approval: 30.06.2022
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Hexal AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TorsemedDosage form: Tablets, 5 mgActive substance: torasemideManufacturer: Polfarmex S.A.Prescription requiredDosage form: Tablets, 10 mgActive substance: torasemideManufacturer: Polfarmex S.A.Prescription requiredDosage form: Tablets, 20 mgActive substance: torasemideManufacturer: Polfarmex S.A.Prescription required
Alternatives to Torsemed in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Torsemed in Spain
Alternative to Torsemed in Ukraine
Online doctors for Torsemed
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Torsemed – subject to medical assessment and local rules.










