
Torendo Q-tab 1 mg

Ask a doctor about a prescription for Torendo Q-tab 1 mg

How to use Torendo Q-tab 1 mg
Package Leaflet: Information for the Patient
Torendo Q-Tab 1 mg, orally disintegrating tablets
Torendo Q-Tab 2 mg, orally disintegrating tablets
Risperidone
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, as you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Torendo Q-Tab and what is it used for
- 2. Important information before taking Torendo Q-Tab
- 3. How to take Torendo Q-Tab
- 4. Possible side effects
- 5. How to store Torendo Q-Tab
- 6. Contents of the pack and other information
1. What is Torendo Q-Tab and what is it used for
Torendo Q-Tab belongs to a group of medicines called antipsychotics.
Torendo Q-Tab is used for:
- Treating schizophrenia - a condition where the patient may see, hear, or feel things that do not exist, believe in untrue things, or experience unusual suspicion or confusion.
- Treating manic episodes - a condition where the patient may feel highly excited, agitated, or overly active. Manic episodes occur in bipolar affective disorder.
- Short-term treatment (up to 6 weeks) of persistent aggression in patients with Alzheimer's dementia who harm themselves or others. Before taking the medicine, alternative non-pharmacological treatment methods should be used.
- Short-term treatment (up to 6 weeks) of persistent aggression in intellectually disabled children (aged 5 and above) and adolescents with behavioral disorders.
Torendo Q-Tab alleviates the symptoms of the disease and prevents their recurrence.
2. Important information before taking Torendo Q-Tab
When not to take Torendo Q-Tab
- If the patient is allergic to risperidone or any of the other ingredients of this medicine (listed in section 6).
If the patient is unsure whether the above information applies to them, they should consult a doctor or pharmacist before taking Torendo Q-Tab.
Warnings and precautions
Before taking Torendo Q-Tab, the patient should contact their doctor or pharmacist:
- If the patient has heart problems, such as an irregular heartbeat, or if the patient has a tendency to low blood pressure, or if the patient is taking blood pressure-lowering medicines. Torendo Q-Tab may cause a decrease in blood pressure. It may be necessary to adjust the dose of the medicine.
- If the patient is aware of any factors that may predispose them to stroke, such as high blood pressure, cardiovascular disorders, or cerebral vascular disorders.
- If the patient has ever experienced involuntary rhythmic movements of the tongue, mouth, or face.
- If the patient has ever experienced a condition characterized by fever, severe muscle stiffness, sweating, or decreased level of consciousness (also known as malignant neuroleptic syndrome).
- In patients with Parkinson's disease or dementia.
- If the patient has had a low white blood cell count in the past (which may or may not have been caused by the action of other medicines).
- In patients with diabetes.
- In patients with epilepsy.
- If the male patient has experienced a prolonged or painful erection.
- If the patient has difficulty controlling body temperature or overheating.
- If the patient has kidney problems.
- If the patient has liver problems.
- If the patient has been found to have an abnormally high level of prolactin in the blood or a suspected prolactin-dependent tumor.
- If the patient or their family members have a history of blood clots, as the use of such medicines as Torendo Q-Tab is associated with the formation of blood clots.
If the patient is unsure whether any of the above conditions apply to them, they should consult a doctor or pharmacist before taking Torendo Q-Tab.
The doctor may order a white blood cell count test, as very rarely, patients taking risperidone have been observed to have a dangerously low number of a certain type of white blood cell necessary for fighting infections.
Torendo Q-Tab may cause weight gain. Significant weight gain can have a negative impact on the patient's health. The doctor should regularly monitor the patient's weight.
The doctor will monitor whether the patient experiences symptoms of high blood sugar, as cases of diabetes or worsening of existing diabetes have been reported in patients taking Torendo Q-Tab. In patients with pre-existing diabetes, blood glucose levels should be regularly monitored.
Torendo Q-Tab often increases the level of a hormone called "prolactin". This can cause side effects such as menstrual disorders, fertility problems in women, breast swelling in men, (see Possible side effects). If such side effects occur, it is recommended to perform a prolactin level test in the blood.
During cataract surgery, the pupil may not dilate sufficiently. The iris may also be floppy during the procedure, which can result in eye damage. If the patient is scheduled for eye surgery, they should inform their ophthalmologist about taking this medicine.
Elderly patients with dementia
In elderly patients with dementia, there is an increased risk of stroke. Patients with dementia caused by stroke should not take risperidone.
During treatment with Torendo Q-Tab, the patient should frequently consult their doctor.
Medical help should be sought immediately if the patient or their caregiver notices a sudden change in the patient's mental state or sudden weakness or numbness of the face, arms, or legs, especially if it is one-sided, or speech disorders, even if they occur for a short time. These symptoms may signal a stroke.
Children and adolescents
Before starting treatment for behavioral disorders, other causes of aggressive behavior should be ruled out.
If during treatment the patient experiences fatigue, concentration may improve by changing the time of risperidone administration.
Before starting treatment, the doctor may check the patient's weight and may regularly monitor the patient's weight during treatment.
In a small study, an increase in growth was observed in children taking Torendo Q-Tab, but it is not known whether this is due to the action of the medicine or other factors.
Torendo Q-Tab and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Patients should especially inform their doctor or pharmacist if they are taking any of the following medicines:
- Medicines that act on the brain, such as sedatives (benzodiazepines) or certain painkillers (opioids), antihistamines, as risperidone may enhance their sedative effect,
- Medicines that may cause a change in the electrical activity of the heart, such as anti-malarial medicines, anti-arrhythmic medicines, antihistamines, or certain antidepressants or other medicines used to treat mental disorders,
- Medicines that slow down the heart rate,
- Medicines that decrease the level of potassium in the blood (e.g., certain diuretics),
- Medicines used to treat high blood pressure, as Torendo Q-Tab may lower blood pressure,
- Medicines used to treat Parkinson's disease (e.g., levodopa),
- Medicines that increase the activity of the central nervous system (psychostimulants, such as methylphenidate,
- Diuretics (used in patients with heart disease or to relieve swelling in areas where there is excessive fluid accumulation, e.g., furosemide or chlorthiazide). Torendo Q-Tab taken alone or in combination with furosemide may increase the risk of stroke or death in elderly patients with dementia.
The following medicines may decrease the effect of risperidone:
- Rifampicin (a medicine used to treat certain infections),
- Carbamazepine, phenytoin (anti-epileptic medicines),
- Phenobarbital. If the patient starts or stops taking these medicines, it may be necessary to adjust the dose of risperidone.
The following medicines may enhance the effect of risperidone:
- Quinidine (used in certain heart diseases),
- Antidepressants, such as paroxetine, fluoxetine, tricyclic antidepressants,
- Beta-adrenolytics (used to treat high blood pressure),
- Phenothiazines (e.g., used to treat psychoses or to sedate),
- Cimetidine, ranitidine (reducing stomach acid),
- Itraconazole or ketoconazole (used in fungal infections),
- Certain medicines used to treat HIV/AIDS, such as ritonavir,
- Verapamil, used to treat high blood pressure and/or arrhythmias,
- Sertraline and fluvoxamine, used to treat depression and other mental disorders.
If the patient starts or stops taking these medicines, it may be necessary to adjust the dose of risperidone.
If the patient is unsure whether they have taken or are taking any of the above medicines, they should consult their doctor or pharmacist before taking Torendo Q-Tab.
Torendo Q-Tab with food, drinks, and alcohol
This medicine can be taken with or without food. During treatment with Torendo Q-Tab, the patient should avoid drinking alcohol.
Pregnancy, breastfeeding, and fertility
- If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking this medicine. The doctor will decide whether the patient can take Torendo Q-Tab.
- In newborns whose mothers took risperidone in the last trimester of pregnancy (the last 3 months of pregnancy), the following symptoms may occur: trembling, muscle stiffness, and/or weakness, drowsiness, agitation, breathing difficulties, and feeding problems. If the child experiences any of these symptoms, they should contact their doctor.
- Torendo Q-Tab may increase the level of prolactin in the blood - a hormone that can affect fertility (see Possible side effects).
Driving and using machines
During treatment with Torendo Q-Tab, the patient may experience dizziness, drowsiness, and vision problems. Therefore, without consulting their doctor, the patient should not drive vehicles or operate machines.
Torendo Q-Tab contains aspartame (E 951)
This medicine contains 0.80 mg of aspartame in each 1 mg orally disintegrating tablet and 1.60 mg of aspartame in each 2 mg orally disintegrating tablet. Aspartame is a source of phenylalanine. It may be harmful to patients with phenylketonuria. This is a rare genetic disorder in which phenylalanine accumulates in the body due to its improper excretion.
3. How to take Torendo Q-Tab
This medicine should always be taken as directed by the doctor or pharmacist. In case of doubts, the patient should consult their doctor or pharmacist.
It should be remembered that not all strengths of these medicinal products are available on the market or not all recommended doses can be achieved with these medicinal products. Other pharmaceutical forms/strengths may be available on prescription.
Recommended doses are given below.
In the treatment of schizophrenia
Adults
- Typically, the initial dose is 2 mg per day, which may be increased to 4 mg on the second day.
- Depending on the patient's response to treatment, the doctor may adjust the dose.
- For most patients, the optimal daily dose is between 4 mg and 6 mg.
- This total daily dose may be given as a single dose or divided into two doses. The doctor will inform the patient which method of administration is best for them.
Elderly patients
- The initial dose is usually 0.5 mg twice a day.
- Then, the doctor may gradually increase the dose to 1 mg to 2 mg twice a day.
- The doctor will inform the patient which method of administration is best for them.
In the treatment of manic episodes
Adults
- The initial dose is usually 2 mg once a day.
- Depending on the patient's response to treatment, the doctor may gradually adjust the dose.
- For most patients, the optimal dose is between 1 mg and 6 mg once a day.
Elderly patients
- The initial dose is usually 0.5 mg twice a day.
- Then, the doctor may gradually adjust the dose to 1 mg to 2 mg twice a day, depending on the patient's response to treatment.
In the treatment of persistent aggression in patients with Alzheimer's dementia
Adults (including elderly patients)
- The initial dose is usually 0.25 mg twice a day.
- Depending on the patient's response to treatment, the doctor may gradually adjust the dose.
- For most patients, the optimal daily dose is 0.5 mg twice a day. Some patients may need a dose of 1 mg twice a day.
- The duration of treatment in patients with Alzheimer's dementia should not exceed 6 weeks.
Children and adolescents
- In children and adolescents under 18 years of age, Torendo Q-Tab should not be used to treat mania in bipolar affective disorder.
In the treatment of behavioral disorders
The dose depends on the child's body weight:
In children with a body weight below 50 kg
- The initial dose is usually 0.25 mg once a day.
- The dose may be increased every other day by 0.25 mg per day.
- Typically, the maintenance dose is between 0.25 mg and 0.75 mg once a day.
In children with a body weight of 50 kg or more
- The initial dose is usually 0.5 mg once a day.
- The dose may be increased every other day by 0.5 mg per day.
- Typically, the maintenance dose is between 0.5 mg and 1.5 mg once a day.
The duration of treatment in patients with behavioral disorders should not exceed 6 weeks.
In children under 5 years of age, Torendo Q-Tab should not be used to treat behavioral disorders.
Patients with renal or hepatic impairment
Regardless of the disease being treated, all initial and subsequent doses should be reduced by half. In these patients, dose increases should be slower.
Risperidone should be used with caution in this group of patients.
Method of administration
Oral administration
Torendo Q-Tab orally disintegrating tablets are fragile. They should not be pressed out of the blister pack, as this may damage them. To remove a tablet from the packaging, the patient should:
- 1. Hold the blister pack by the edges and separate the square containing the tablet from the rest of the blister pack. This should be done gently along the perforation.
- 2. Pull the edge of the foil and tear it off completely.
- 3. Shake the tablet onto the patient's hand.
- 4. Place the tablet on the tongue immediately after removing it from the packaging.
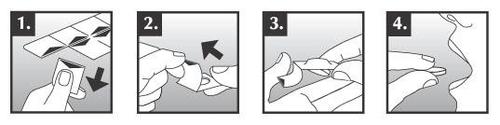
After a few seconds, the tablet will start to disintegrate in the mouth and can be swallowed, with or without water. The patient should not have anything in their mouth before placing the tablet on their tongue.
The tablet can also be placed in a glass or cup of water and taken immediately.
Overdose of Torendo Q-Tab
- The patient should immediately consult their doctor. They should take the packaging with the medicine.
- In case of overdose, the patient may experience drowsiness or fatigue, or abnormal body movements, difficulty standing or walking, dizziness due to low blood pressure, or an abnormal heart rhythm, or may experience a seizure.
Missed dose of Torendo Q-Tab
- If the patient forgets to take a dose, they should take the next dose as soon as possible. However, if it is almost time for the next dose, they should skip the missed dose and take the next dose as directed. If the patient misses two or more doses, they should consult their doctor.
- The patient should not take a double dose (two doses at the same time) to make up for a missed dose.
Stopping treatment with Torendo Q-Tab
The patient should not stop taking the medicine unless it is in agreement with their doctor. It is possible that the symptoms of the disease will recur. If the doctor decides to discontinue treatment, the dose of the medicine may be gradually reduced over several days.
In case of any further doubts about the use of this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The patient should immediately inform their doctor if they experience any of the following uncommon side effects (may affect up to 1 in 100 people):
- In a patient with dementia, a sudden change in mental state or sudden weakness or numbness of the face, arms, or legs, especially if it is one-sided, or speech disorders, even if they occur for a short time. These symptoms may signal a stroke.
- The patient experiences late dyskinesia (involuntary, rhythmic, or jerky movements of the face, tongue, or other parts of the body). The patient should immediately consult their doctor if they experience involuntary rhythmic movements of the tongue, mouth, or face. It may be necessary to discontinue risperidone.
The patient should immediately inform their doctor if they experience any of the following rare side effects (may affect less than 1 in 1,000 people):
- The patient experiences blood clots in the veins, especially in the legs (symptoms include pain and redness of the leg), which can move through the blood vessels to the lungs, causing chest pain and breathing difficulties. If such symptoms occur, the patient should immediately seek medical help.
- The patient experiences fever, severe muscle stiffness, sweating, or decreased level of consciousness (a condition known as malignant neuroleptic syndrome). Immediate treatment may be necessary.
- The male patient experiences a prolonged or painful erection. This condition is known as priapism. Immediate treatment may be necessary.
- The patient experiences a severe allergic reaction characterized by fever, swelling of the lips, face, tongue, or throat, difficulty breathing, itching, rash, or a drop in blood pressure.
The following side effects may occur:
Very common side effects (may affect more than 1 in 10 people):
- Difficulty sleeping or waking up.
- Parkinsonism: This condition may include slow or abnormal movements, a feeling of stiffness or tension in the muscles (which can make the patient's movements uneven, jerky), and sometimes even a feeling of "freezing" of movements, followed by a release. Other symptoms of parkinsonism include a slow, shuffling gait, tremors, increased salivation, and a mask-like face.
- Feeling drowsy or less alert.
- Headache.
Common side effects (may affect up to 1 in 10 people):
- Pneumonia, bronchitis, symptoms of a cold, sinusitis, urinary tract infection, ear infection, flu-like symptoms.
- Urinary tract infection, ear infection, flu-like symptoms.
- Increased level of prolactin in the blood (with or without symptoms). Symptoms of increased prolactin levels, which occur uncommonly, may include in men: breast swelling, difficulty achieving or maintaining an erection, decreased sex drive, or other sexual disorders. In women, they may include breast discomfort, milk secretion from the breasts, absence of menstruation, or other menstrual disorders or fertility problems.
- Weight gain, increased appetite, decreased appetite.
- Sleep disorders, irritability, depression, anxiety, restlessness.
- Dystonia: In this condition, there are slow or sustained involuntary muscle contractions. Although it can affect any part of the body (which can result in an abnormal posture), dystonia most commonly affects the muscles of the face, including abnormal movements of the eyes, mouth, tongue, or jaw.
- Dizziness.
- Dyskinesias: In this condition, there are involuntary muscle movements, including repetitive, spasmodic, or twisting movements or jerks.
- Tremors.
- Blurred vision, eye infection, or conjunctivitis.
- Rapid heartbeat, high blood pressure, shortness of breath (dyspnea).
- Sore throat, cough, nosebleed, stuffy nose.
- Abdominal pain, discomfort in the abdomen, vomiting, nausea, constipation, diarrhea, indigestion, dry mouth, toothache.
- Rash, redness of the skin.
- Muscle spasms, muscle or bone pain, back pain, joint pain.
- Urinary incontinence.
- Swelling of the body, arms, or legs, fever, chest pain, weakness, fatigue, pain.
- Falls.
Uncommon side effects (may affect up to 1 in 100 people):
- Respiratory tract infection, urinary tract infection, eye infection, tonsillitis, fungal infection of the nails, skin infection, skin infection limited to one area or part of the body, viral infection, skin inflammation caused by mites.
- Decreased white blood cell count (including those that help protect against infections), decreased platelet count (blood cells that help stop bleeding), anemia, decreased red blood cell count, increased eosinophil count (a type of white blood cell).
- Allergic reaction.
- Onset of diabetes or worsening of existing diabetes, high blood sugar, excessive thirst.
- Weight loss, loss of appetite leading to malnutrition and low body weight.
- High cholesterol levels in the blood.
- Elevated mood (mania), confusion, decreased libido, nervousness, nightmares.
- Lack of reaction to stimuli, loss of consciousness, low level of consciousness.
- Seizures, fainting.
- Need to move body parts, balance disorders, coordination disorders, dizziness when changing position to standing, concentration disorders, speech difficulties, loss of or abnormal taste, numbness or tingling sensation on the skin.
- Increased sensitivity of the eyes to light, dry eye, increased tearing, redness of the eyes.
- Feeling of dizziness, ringing in the ears, ear pain.
- Atrial fibrillation (abnormal heart rhythm), conduction disorder between the heart chambers, abnormal conduction of electrical impulses in the heart, prolonged QT interval, slow heartbeat, abnormal electrocardiogram (ECG), feeling of palpitations.
- Low blood pressure, low blood pressure when changing position to standing (which may cause some patients taking Torendo Q-Tab to faint, feel dizzy, or lose consciousness), sudden flushing of the skin, especially the face.
- Aspiration pneumonia (caused by food entering the airways), pulmonary congestion, wheezing in the lungs, hoarseness (voice disorder), breathing difficulties.
- Gastrointestinal infection, diarrhea, very hard stools, difficulty swallowing, excessive gas.
- Hives, itching, hair loss, skin thickening, rash, dry skin, skin discoloration, acne, flaky, itchy skin on the head or body, skin disorders, skin damage.
- Increased CPK (creatine phosphokinase) activity in the blood, an enzyme that is sometimes released from damaged muscles.
- Abnormal posture, joint stiffness, joint swelling, muscle weakness, neck pain.
- Frequent urination, urinary incontinence, painful urination.
- Erectile dysfunction, ejaculation disorders.
- Absence of menstruation, irregular menstrual cycle, and other menstrual disorders (in women).
- Breast swelling in men, milk secretion from the breasts, sexual disorders, breast pain, breast discomfort, vaginal discharge.
- Swelling of the face, lips, eyes, or tongue.
- Chills, increased body temperature.
- Change in gait.
- Thirst, malaise, chest discomfort, feeling unwell, discomfort.
- Increased activity of aminotransferases in the blood, increased activity of the enzyme GGTP (liver enzyme - gamma-glutamyltransferase) in the blood, increased activity of liver enzymes in the blood.
- Pain related to medical procedures.
Rare side effects (may affect less than 1 in 1,000 people):
- Infections.
- Abnormal secretion of the hormone that regulates the amount of urine.
- Sleepwalking (somnambulism).
- Eating disorders related to sleep.
- Sugar in the urine, low blood sugar, high triglyceride levels (fats) in the blood.
- Lack of emotions, inability to achieve orgasm.
- Decreased motor activity and lack of response in a patient with preserved consciousness (catatonia).
- Cerebrovascular disorders.
- Coma due to uncontrolled diabetes.
- Shaking or nodding movements of the head.
- Glaucoma (increased pressure in the eyeball), eye movement disorders, rotational eye movements, ulcers on the edges of the eyelids.
- Complications during cataract surgery. During this procedure, a condition called intraoperative floppy iris syndrome (IFIS) may occur if the patient is taking or has taken Torendo Q-Tab. If the patient is scheduled for cataract surgery, they should inform their ophthalmologist about taking this medicine.
- Dangerously low number of certain white blood cells responsible for fighting infections.
- Dangerously excessive thirst.
- Irregular heartbeat.
- Sleep apnea (breathing difficulties during sleep), rapid, shallow breathing.
- Pancreatitis, intestinal obstruction.
- Tongue swelling, lip swelling, drug rash.
- Dandruff.
- Muscle fiber breakdown and muscle pain (rhabdomyolysis).
- Delayed menstruation, breast swelling, breast enlargement, milk secretion from the breasts.
- Increased insulin levels in the blood (a hormone that regulates blood sugar levels).
- Skin hardening.
- Low body temperature, cooling of the hands and feet.
- Withdrawal symptoms.
- Jaundice (yellowing of the skin and eyes).
Very rare side effects (may affect less than 1 in 10,000 people):
- Life-threatening complications related to uncontrolled diabetes.
- A severe allergic reaction with swelling that can affect the throat and cause breathing difficulties.
- Intestinal obstruction leading to bowel obstruction.
Side effects with unknown frequency: the frequency cannot be estimated from the available data:
- A severe or life-threatening rash with blisters and peeling skin, which can occur in the mouth, nose, eyes, and genitals, and may also spread to other parts of the body (Stevens-Johnson syndrome or toxic epidermal necrolysis).
Side effects observed during the use of another medicine - paliperidone, which is very similar to risperidone, which may also occur during the use of Torendo Q-Tab:
rapid heartbeat when changing position to standing.
Additional side effects in children and adolescents
It is assumed that the side effects in children will be similar to those in adults.
The following side effects were more common in children and adolescents (aged 5 to 17) than in adults: drowsiness or decreased alertness, fatigue, headache, increased appetite, vomiting, cold symptoms, runny nose, abdominal pain, dizziness, cough, fever, tremors, diarrhea, and urinary incontinence.
Reporting side effects
If side effects occur, including those not listed in this leaflet, the patient should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Torendo Q-Tab
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
There are no special storage precautions.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Torendo Q-Tab contains
- The active substance of the medicine is risperidone. Each orally disintegrating tablet contains 1 mg or 2 mg of risperidone.
- The other ingredients are: mannitol, methacrylic acid copolymer, povidone, microcrystalline cellulose, low-substituted hydroxypropylcellulose, aspartame (E 951), crospovidone, red iron oxide (E 172), peppermint flavor, spearmint flavor, calcium silicate, magnesium stearate. See section 2 "Torendo Q-Tab contains aspartame (E 951)".
What Torendo Q-Tab looks like and contents of the pack
The orally disintegrating tablets are round, slightly convex, pink, and mottled.
Packaging:20, 28, 30, 50, 56, 60, 98, or 100 orally disintegrating tablets in blister packs, in a cardboard box
Marketing authorization holder
KRKA, d.d., Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Slovenia
Manufacturer
KRKA, d.d., Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Slovenia
To obtain more detailed information on the names of the medicine in other EU member states and the UK (Northern Ireland), the patient should contact the local representative of the marketing authorization holder:
KRKA-POLSKA Sp. z o.o.
Równoległa 5
02-235 Warsaw
Phone: 22 57 37 500
Date of last revision of the leaflet: 27.03.2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterKrka, d.d., Novo mesto
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Torendo Q-tab 1 mgDosage form: Solution, 1 mg/mlActive substance: risperidonePrescription requiredDosage form: Tablets, 1 mgActive substance: risperidoneManufacturer: Orion Corporation Orion CorporationPrescription requiredDosage form: Tablets, 2 mgActive substance: risperidoneManufacturer: Orion Corporation Orion CorporationPrescription required
Alternatives to Torendo Q-tab 1 mg in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Torendo Q-tab 1 mg in Spain
Alternative to Torendo Q-tab 1 mg in Ukraine
Online doctors for Torendo Q-tab 1 mg
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Torendo Q-tab 1 mg – subject to medical assessment and local rules.









