

Tisseel Lio

Ask a doctor about a prescription for Tisseel Lio

How to use Tisseel Lio
Leaflet accompanying the packaging: information for the user
TISSEEL Lyo - powders and solvents for preparing tissue glue
Human fibrinogen, human thrombin, synthetic aprotinin, calcium chloride dihydrate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is TISSEEL Lyo and what is it used for
- 2. Important information before using TISSEEL Lyo
- 3. How to use TISSEEL Lyo
- 4. Possible side effects
- 5. How to store TISSEEL Lyo
- 6. Contents of the packaging and other information
1. What is TISSEEL Lyo and what is it used for
What is TISSEEL Lyo
TISSEEL Lyo is a two-component tissue glue consisting of two solutions, a solution of clotting proteins and a solution of thrombin. TISSEEL Lyo contains fibrinogen and thrombin. These are two blood proteins necessary for blood clotting. When these proteins are mixed during administration, they form a clot at the site where the surgeon applies them.
What is TISSEEL Lyo used for
TISSEEL Lyo is used as an adjunctive treatment when standard surgical techniques are not sufficiently effective: to improve hemostasis; as a tissue glue, facilitating wound healing or as a sealing material for sutures in vascular and gastrointestinal surgery; for tissue adhesion, e.g., fixation of skin transplants. TISSEEL Lyo is also effective in patients receiving heparinized blood.
2. Important information before using TISSEEL Lyo
When not to use TISSEEL Lyo:
- In case of allergy (hypersensitivity) to any of the active substances or any of the other components of this medicine.
- For the treatment of massive arterial or venous bleeding. The use of TISSEEL Lyo alone is not indicated in such a situation.
- TISSEEL Lyo must not be injected into blood vessels (veins or arteries). Because TISSEEL Lyo forms clots at the site of administration, injecting TISSEEL Lyo may cause a clot to form in the vessel. If such a clot is carried by the blood, it may lead to life-threatening complications.
- TISSEEL Lyo is not intended to replace skin sutures used to close a surgical wound.
Warnings and precautions
Before starting treatment with TISSEEL Lyo, discuss it with your doctor, pharmacist, or nurse. Particular care should be taken when using TISSEEL Lyo due to the possibility of allergic hypersensitivity reactions. The first signs of an allergic reaction may include: transient skin redness, itching, rash, nausea, vomiting, general malaise, chills, chest tightness, swelling of the lips and tongue, breathing difficulties / apnea, drop in blood pressure, increase or decrease in heart rate.
- Because TISSEEL Lyo contains a synthetic protein called aprotinin. Even if this protein is administered only in small amounts and only to the surface of the wound, there is a risk of a severe anaphylactic reaction. This risk appears to be higher in patients who have previously been treated with TISSEEL Lyo or aprotinin, even if it was previously well tolerated. Therefore, information about the use of aprotinin or products containing aprotinin should be noted in the patient's medical history. Since synthetic aprotinin is structurally identical to bovine aprotinin, the use of TISSEEL Lyo should be carefully considered in patients with an allergy to bovine proteins.
- Because accidental intravascular administration may lead to life-threatening complications due to the entry of the formed clot into the bloodstream.
- Intravascular administration, especially in sensitive patients, may increase the likelihood and severity of acute hypersensitivity reactions. In particular, during cardiothoracic surgery, the surgeon must exercise particular caution to avoid injecting TISSEEL Lyo into a blood vessel. It is also important to avoid injection into the nasal mucosa due to the risk of blood clots in the arteries of the eye area.
- Because there is a risk of local tissue damage in case of injection of TISSEEL Lyo into tissue.
- To avoid gluing tissues in an unwanted location. Therefore, before application, attention should be paid to whether the body areas outside the target application site are sufficiently protected.
- Because too great a thickness of the formed clot may adversely affect the effectiveness of the product and the wound healing process. Therefore, TISSEEL Lyo should only be applied in a thin layer.
Caution should be exercised when applying fibrin glue using compressed gas.
During the use of spray devices equipped with a pressure regulator for applying fibrin glues to tissues, very rare cases of life-threatening or fatal air and gas embolism have been reported (involving the entry of air into the bloodstream, which can lead to serious health risks or death). It appears to be related to the use of the spray device at higher than recommended pressure settings and (or) very close to the tissue surface. The risk appears to be higher when fibrin glues are sprayed with air than when they are sprayed with CO2. For this reason, it cannot be ruled out that such an event may occur as a result of spraying the TISSEEL Lyo product onto surgically treated open wounds.
Instructions for use and recommendations from the manufacturer regarding pressure ranges and distance from the tissue surface during spraying are attached to the spray device and additional nozzle.
The TISSEEL Lyo product should be administered strictly according to the instructions and only using devices recommended for use with this product.
During spraying of the TISSEEL Lyo product, monitor changes in arterial blood pressure, heart rate, arterial blood oxygen saturation, and end-expiratory CO2 concentration due to the possibility of air or gas embolism (see section 2).
In the manufacture of medicines from human blood or plasma, certain measures are taken to prevent the transmission of infections to patients. These include: careful selection of blood and plasma donors to ensure that risk groups for infection are excluded; testing of individual blood donations and pooled plasma for viruses or infections; inclusion of steps in the blood or plasma processing process that can inactivate or remove viruses.
- careful selection of blood and plasma donors to ensure that risk groups for infection are excluded
- testing of individual blood donations and pooled plasma for viruses or infections
- inclusion of steps in the blood or plasma processing process that can inactivate or remove viruses
Despite these measures, when medicines made from human blood or plasma are administered, the possibility of transmitting an infection cannot be completely ruled out. This also applies to unknown or newly discovered viruses and other pathogens. The measures taken are considered effective against enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, as well as non-enveloped hepatitis A virus. The measures taken may have limited value against some non-enveloped viruses, such as parvovirus B19. Parvovirus B19 infection can be dangerous for pregnant women (fetal infection) and for people with weakened immune systems or with certain types of anemia (e.g., congenital spherocytosis or hemolytic anemia). The attending physician may recommend that the patient consider vaccination against viral hepatitis A and B if the patient is repeatedly receiving fibrin glue. It is strongly recommended that each administration of TISSEEL Lyo be documented with the name and batch number of the medicine using the attached self-adhesive label, which should be placed in the patient's medical history.
TISSEEL Lyo and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, even those available without a prescription. No interactions with other medicinal products are known. Like other comparable preparations or thrombin solutions, the product may denature under the influence of solutions containing alcohol, iodine, or heavy metals (e.g., disinfectant solutions). Before applying the product, the surface should be cleaned as thoroughly as possible of such substances. Information about preparations containing oxidized cellulose can be found in the "Preparation for use" section.
TISSEEL Lyo with food and drink
Ask your doctor. The doctor will decide whether the patient can eat and drink before using TISSEEL Lyo.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist before using this medicine. The doctor will decide whether TISSEEL Lyo can be used during pregnancy or breastfeeding.
Driving and using machines
TISSEEL Lyo has no effect on the ability to drive vehicles or operate machinery.
TISSEEL Lyo contains Polysorbate 80
Polysorbate 80 may cause local skin reactions, such as contact dermatitis.
3. How to use TISSEEL Lyo
The TISSEEL Lyo product can only be used by experienced surgeons who have been trained in this regard. Before applying the TISSEEL Lyo product, the wound surface should be dried using standard techniques (e.g., changing compresses, gauzes, using suction devices). Compressed air or gas should not be used to dry the surface. TISSEEL Lyo can only be sprayed on visible surfaces.
When applying the TISSEEL Lyo product using a spray device, follow the manufacturer's recommendations for pressure and distance from the tissue in accordance with the ranges specified below:
| Recommended pressure and distance from tissue values and spray device for applying the TISSEEL Lyo product | |||||
| Surgical treatment | Spray set to be used | Aplier tips to be used | Pressure regulator to be used | Recommended distance from target tissue | Recommended spray pressure |
| Open wound | Tisseel/Artiss spray set | nd. | EasySpray | 10–15 cm | 1.5–2.0 bar (21.5–28.5 psi) |
| Tisseel/Artiss spray set - 10-piece package | nd. | EasySpray | |||
| Laparoscopic/minimally invasive procedures | nd. | Duplospray MIS 20 cm applicator | Duplospray MIS 1.5 bar pressure regulator | 2–5 cm | 1.2–1.5 bar (18–22 psi) |
| Duplospray MIS 30 cm applicator | |||||
| Duplospray MIS 40 cm applicator | |||||
| Spray Set 360 endoscopic applicator with Snap Lock | |||||
| Spray Set 360 endoscopic applicator with safety strap | |||||
| Exchangeable nozzle | |||||
During spraying of the TISSEEL Lyo product, monitor changes in arterial blood pressure, heart rate, arterial blood oxygen saturation, and end-expiratory CO2 concentration due to the possibility of air or gas embolism (see section 2).
The dose of the applied glue is always determined based on individual needs. The dose depends on a number of factors, including the type of surgical procedure, the size of the glued surface, the chosen method of applying the glue, and the number of applications. The doctor will decide what amount is needed and apply a thin, even layer to the target surface. If the applied amount does not seem sufficient, the glue application can be repeated. When applying the TISSEEL Lyo product, a clot forms quickly. Avoid applying a new layer on an existing layer of TISSEEL Lyo, as the new layer will not bond with the existing one. It is absolutely necessary to avoid separate application of the clotting protein component and the thrombin component. In clinical trials, individually adjusted doses administered ranged from 4 to 20 ml. In some procedures (e.g., traumatic liver injury or treatment of extensive burn wounds), larger volumes may be required. As a guideline for gluing surfaces, it can be assumed that 1 set of TISSEEL Lyo glue 2 ml (i.e., 1 ml of Tisseel solution plus 1 ml of thrombin solution) is sufficient to cover an area of at least 10 cm². When TISSEEL Lyo is applied by spraying, the same amount will be sufficient to cover larger surfaces. To avoid excessive granulation and to ensure gradual absorption of the solidified glue into the tissues, it is recommended to apply the thinnest possible layers of TISSEEL Lyo. To ensure proper mixing of the clotting protein solution and thrombin solution, it is recommended to squeeze out the first few drops of the product from the application needle and immediately discard them before applying the product.
Overdose of TISSEEL Lyo
TISSEEL Lyo is used only during surgical operations. The doctor determines the amount needed to be applied. No cases of overdose are known. If you have any questions about using the medicine, ask your doctor or pharmacist.
Use in children
The safety and efficacy of using the medicine in children have not been established.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. In patients treated with tissue glue, hypersensitivity reactions or allergic reactions may occur. Although they are very rare, they can be very serious. The first signs of an allergic reaction may include: transient skin redness, itching, rash, nausea, vomiting, headache, drowsiness, restlessness, burning or stinging at the injection site, ringing in the ears, chills, chest tightness, swelling of the lips, tongue, and throat (which may cause difficulty breathing and/or swallowing), breathing difficulties, low blood pressure, increase or decrease in heart rate, loss of consciousness due to a drop in blood pressure.
- transient skin redness
- itching
- rash
- nausea, vomiting
- headache
- drowsiness
- restlessness
- burning or stinging at the injection site
- ringing in the ears
- chills
- chest tightness
- swelling of the lips, tongue, and throat (which may cause difficulty breathing and/or swallowing)
- breathing difficulties
- low blood pressure
- increase or decrease in heart rate
- loss of consciousness due to a drop in blood pressure
In individual cases, these reactions can develop into severe allergic reactions (anaphylaxis). Such reactions can occur especially when the preparation is used repeatedly or is administered to patients who have previously shown hypersensitivity to aprotinin or any other component of the product. Even if previous repeated use of TISSEEL Lyo was well tolerated, subsequent administration of TISSEEL Lyo or injection of aprotinin may lead to severe allergic (anaphylactic) reactions. The surgical team is aware of the risk of this type of reaction, and if the first signs of hypersensitivity appear, the administration of TISSEEL Lyo will be stopped immediately. Severe symptoms may require emergency measures. Injection of TISSEEL Lyo into soft tissues may cause local tissue damage. Injection of TISSEEL Lyo into blood vessels (veins or arteries) may cause clot formation (thrombosis). Since TISSEEL Lyo is made from plasma derived from human blood, the risk of infection cannot be completely ruled out. Nevertheless, the manufacturer takes numerous measures to reduce this risk (see section 2). In rare cases, antibodies against the components of the tissue glue may appear.
The following side effects have been observed with TISSEEL Lyo:
Side effects were assessed according to the following frequency categories: Very common: may affect more than 1 in 10 people; Common: may affect up to 1 in 10 people; Uncommon: may affect up to 1 in 100 people; Rare: may affect up to 1 in 1,000 people; Very rare: may affect up to 1 in 10,000 people; Not known: frequency cannot be estimated from the available data.
| Location | Side effect | Frequency |
| Infections and infestations | Postoperative wound infection | Common |
| Blood and lymphatic system disorders | Increased fibrin degradation products | Uncommon |
| Immune system disorders | Hypersensitivity reactions | Uncommon |
| Allergic reactions (anaphylactic) | Uncommon | |
| Anaphylactic shock | Uncommon | |
| Feeling of ringing, stinging, or numbness of the skin | Uncommon | |
| Chest tightness | Uncommon | |
| Breathing difficulties | Uncommon | |
| Itching | Uncommon | |
| Redness of the skin | Uncommon | |
| Nervous system disorders | Sensory disturbances | Common |
| Cardiac disorders | Increased or decreased heart rate | Uncommon |
| Vascular disorders | Axillary vein thrombosis | Common |
| Low blood pressure | Rare | |
| Bruising | Uncommon | |
| Air or gas bubbles in the vascular system* | Not known | |
| Blood clots in the veins | Uncommon | |
| Cerebral artery occlusion | Uncommon | |
| Respiratory, thoracic, and mediastinal disorders | Dyspnea | Uncommon |
*The occurrence of air or gas bubbles in the vascular system occurred when fibrin glues were applied using devices with compressed air or gas; this appears to be related to incorrect use of the spray device (e.g., at higher than recommended pressure and closer than recommended distance from the tissue surface).
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides of the Office for Registration of Medicinal Products, Medical Devices, and Biocides, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store TISSEEL Lyo
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the label. Store below 25°C. Do not freeze. Store the vials in the outer carton to protect from light. If the reconstituted solutions are not used immediately, they can be stored at 37°C or at room temperature (not exceeding 25°C) without mixing for no longer than 4 hours. Do not expose the TISSEEL Lyo product to temperatures above 37°C, and do not subject it to microwave radiation. Reconstituted solutions should not be refrigerated or frozen. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What TISSEEL Lyo contains
TISSEEL Lyo consists of two components:
Component 1: Clotting protein solution
To obtain the clotting protein solution, the clotting protein concentrate (lyophilized) should be dissolved in the aprotinin solution.
- 1) The active substance of the clotting protein concentrate is: human fibrinogen, 91 mg/ml. Other components are: human albumin, L-histidine, nicotinamide, polysorbate 80 (Tween 80), disodium citrate dihydrate.
1a) The active substance of the aprotinin solution (solvent for the clotting protein concentrate) is synthetic aprotinin 3000 KIU/ml. Another component is water for injections.
Component 2: Thrombin solution
To obtain the thrombin solution, the thrombin (lyophilized) should be dissolved in the calcium chloride solution.
- 2) The active substance of the thrombin (lyophilized) is: human thrombin, 500 IU/ml. Other components are: human albumin and sodium chloride.
2a) The active substance of the calcium chloride solution (solvent for the thrombin powder) is calcium chloride dihydrate, 40 μmol/ml. Another component is water for injections. TISSEEL Lyo contains human factor XIII, copurified with human fibrinogen, in amounts of 0.6–5 IU/ml.
| After mixing | 1 ml | 2 ml | 4 ml | 10 ml |
| Component 1: Clotting protein solution Fibrinogen human (as clotting protein) Synthetic aprotinin | 45.5 mg 1500 KIU | 91 mg 3000 KIU | 182 mg 6000 KIU | 455 mg 15000 KIU |
| Component 2: Thrombin solution Human thrombin Calcium chloride dihydrate | 250 IU 20 μmol | 500 IU 40 μmol | 1000 IU 80 μmol | 2500 IU 200 μmol |
What TISSEEL Lyo looks like and what the pack contains
All components of TISSEEL Lyo are in glass vials. The vial containing the Tisseel powder is equipped with a magnetic stirrer. The lyophilized components are white or slightly yellowish and have a powder or granule consistency, the liquid components are clear, colorless, or slightly yellowish.
Contents of the pack:
1 vial containing Tisseel powder (clotting proteins) (component 1, lyophilized, with 91 mg/ml human fibrinogen)
1 vial containing thrombin powder (component 2, lyophilized, with 500 IU/ml human thrombin)
1 vial containing synthetic aprotinin solution (solvent for component 1 with 3000 KIU/ml aprotinin)
1 vial containing calcium chloride solution (solvent for component 2 with 40 μmol/ml calcium chloride dihydrate)
1 Duploject set for reconstitution and application consisting of: 1 double syringe holder DUPLOJECT, 2 connectors, 2 single-use syringes with blue scale for Tisseel, 2 single-use syringes with black scale for thrombin, 4 single-use needles, 4 application needles (with blunt tip)
Package size:
TISSEEL Lyo is available in the following package sizes: 1 x 2 ml (1 ml + 1 ml), 1 x 4 ml (2 ml + 2 ml), and 1 x 10 ml (5 ml + 5 ml). Not all package types may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Baxter Polska Sp. z o.o., ul. Kruczkowskiego 8, 00-380 Warsaw
Manufacturer:
Takeda Manufacturing Austria AG, Industriestrasse 67, 1221 Vienna, Austria
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: TISSEEL Lyo, Pulver und Lösungsmittel für einen Gewebekleber
Bulgaria: ТИСИЛ ЛИО, прахове и разтворители за тъканно лепило
Czech Republic: TISSEEL Lyo, Prášek pro přípravu tkáňového lepidla rozpouštědlem
Hungary: TISSEEL Lyo, Por és oldószer szövetragasztóhoz
Norway: TISSEEL
Poland: TISSEEL Lyo
Date of last revision of the leaflet: January 2022
---------------------------------------------------------------------------------------------------------------------------
Information intended only for healthcare professionals:
General information
Before applying TISSEEL Lyo, protect the body areas outside the target application site to avoid unwanted tissue adhesion. To prevent the glue from sticking to gloves and instruments, moisten them with a physiological saline solution before coming into contact with the glue. As a guideline for gluing surfaces, it can be assumed that 1 set of TISSEEL Lyo glue 2 ml (i.e., 1 ml of Tisseel solution plus 1 ml of thrombin solution) is sufficient to cover an area of at least 10 cm². The required dose depends on the size of the glued surface.
Preparation for use and reconstitution
Before reconstituting the glue components, clean the rubber stoppers of all vials. Avoid direct contact between disinfectants and the product (see section 4.5). I.
Preparation of the clotting protein solution (component 1)
To obtain the clotting protein solution, dissolve the Tisseel powder in the aprotinin solution. The Tisseel powder can be reconstituted using the FIBRINOTHERM heating and mixing device (recommended method). Alternatively, a sterile water bath at a temperature of 33–37°C can be used. Reconstitution using the FIBRINOTHERM device: The FIBRINOTHERM device maintains a constant temperature of 37°C. It also shortens the dissolution time of the Tisseel powder thanks to the magnetic stirrer located in each vial of Tisseel powder.
- Place the vials containing Tisseel powder and aprotinin solution in the appropriate openings of the FIBRINOTHERM device and heat them for about 3 minutes.
- Transfer the aprotinin solution to the vial containing Tisseel powder using one of the needles and a syringe with a blue scale, which are located in a single, sterile set for reconstituting the product. Place the Tisseel vial in the FIBRINOTHERM device stirrer well (if necessary, use adapter rings) and mix until the product is completely dissolved. Reconstitution is complete when no undissolved particles are visible when viewed against the light. If particles are visible, continue mixing the solution at 37°C for a few more minutes until it is completely dissolved. After reconstitution, turn off the magnetic stirrer.
Note:Do not mix for too long - excessive mixing could decrease the quality of the product!
- If the clotting protein solution is not used immediately, store it at 37°C or at room temperature. Before drawing up the clotting protein solution into the syringe with a blue scale, which is located in the double, sterile set for application, gently swirl the solution to ensure the product's homogeneity.
- Draw up the reconstituted clotting protein solution from the vial under sterile conditions.
Additional instructions are provided in the FIBRINOTHERM device operating instructions. Reconstitution using a water bath: Preheat the vials containing Tisseel powder and aprotinin solution in a water bath at a temperature of 33°C–37°C for about 3 minutes. (Avoid heating above 37°C!) Using one of the needles and a syringe with a blue scale, which are located in a single, sterile set for reconstituting the product, transfer the aprotinin solution to the vial containing Tisseel powder. Place the Tisseel vial back in the water bath at a temperature of 33°C–37°C for 1 minute. Gently swirl the product to avoid excessive foaming. Then, place the vial back in the water bath and check occasionally to see if the product has dissolved completely. Reconstitution is complete when no undissolved particles are visible when viewed against the light. If particles are visible, heat the vial in a water bath at a temperature of 33°C–37°C for a few more minutes and shake it until it is completely dissolved.
- If the clotting protein solution is not used immediately, store it at 33–37°C. Before drawing up the clotting protein solution into the syringe with a blue scale, which is located in the double, sterile set for application, gently swirl the solution to ensure the product's homogeneity.
- Draw up the reconstituted clotting protein solution from the vial under sterile conditions.
Note:When using a water bath for reconstitution instead of the FIBRINOTHERM device, take special precautions to avoid complete immersion of the vial to prevent potential contamination. II.
Preparation of the thrombin solution (component 2)
To obtain the thrombin solution, dissolve the thrombin powder in the calcium chloride solution. Transfer the contents of the vial containing calcium chloride solution to the vial containing thrombin using the second needle and syringe with a black scale, which are located in a single, sterile set for reconstituting the product. Gently swirl to dissolve the lyophilizate. To heat the thrombin solution, the FIBRINOTHERM device or a water bath can be used. Until use, store the thrombin solution at 33°C–37°C. Before use, draw up the thrombin solution from the vial using the second needle and syringe with a black scale, which are located in the double, sterile set for application.
Note:Syringes and needles used for reconstituting one component must not be reused for reconstituting the other components, as this could lead to premature coagulation of that component in the vial or syringe. III.
Use of reconstituted tissue glue components
Both components of the fibrin glue must be used within 4 hours of reconstitution. Reconstituted solutions must not be refrigerated or frozen. The clotting protein solution and thrombin solution should be clear or slightly opalescent. Do not use solutions that are cloudy or contain sediment. Before use, inspect the reconstituted solutions visually for the presence of undissolved particles or changes in color or appearance. If any of these conditions are present, discard the solution. Before administration, warm TISSEEL Lyo to a temperature of 33°C–37°C. Do not expose the TISSEEL Lyo product to temperatures above 37°C. Do not subject it to microwave radiation. To apply the glue, place two single-use syringes filled with the reconstituted clotting protein solution and thrombin solution in the double syringe holder DUPLOJECT, and secure the whole to the connector and application needle. The double, sterile set for application contains all the devices necessary for application.
The common plunger of the double syringe holder DUPLOJECT ensures that equal volumes of both components are delivered to the connector, which are then mixed in the application needle and expelled. Operating instructions
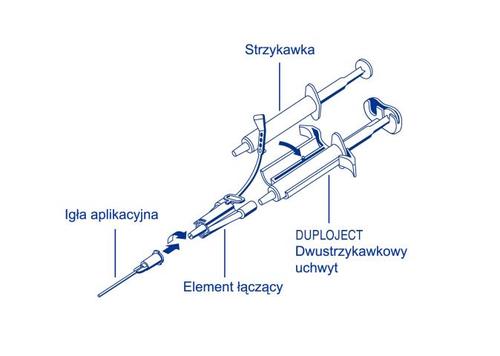
- Place both syringes containing the clotting protein solution and thrombin solution in the holder. Both syringes should be filled with equal volumes.
- Remove all air from the syringe before attaching any application device.
- Position the connector and secure it to the side of the syringe with a safety strap.
- Connect the outlets of both syringes to the connector, ensuring they are properly secured.
- Secure the connector by fastening the safety strap to the double syringe holder DUPLOJECT.
- In the event of a broken safety strap, use the additional connector provided in the package.
- If there is no additional connector, the package can still be used, but ensure that the connection is secure and leak-proof.
- Do not remove air remaining in the connector.
- Attach the application needle to the connector.
- Do not remove air remaining in the connector and application needle before starting the actual application, as this could cause the application needle to become clogged.
- Apply the mixed clotting protein-thrombin solution to the target surface or surfaces to be glued.
Administration
Before applying the TISSEEL Lyo product, dry the wound surface using standard techniques (e.g., changing compresses, gauzes, using suction devices). Compressed air or gas should not be used to dry the surface.
- Apply the mixed clotting protein-thrombin solution to the target surface or surfaces to be glued, slowly pressing the rear part of the common plunger.
- In surgical procedures that require minimal amounts of fibrin glue, it is recommended to express and discard the first few drops of the product.
- After applying TISSEEL Lyo, wait at least 2 minutes to achieve sufficient polymerization.
Note:If the application of the fibrin glue components is interrupted, the needle may become clogged. In such a case, the application needle should be replaced with a new one immediately before reapplying the glue. If the connector outlets are clogged, use the additional connector provided in the package. After mixing the fibrin glue components, coagulation occurs within a few seconds due to the high concentration of thrombin (500 IU/ml). The fibrin glue can also be applied using other additional devices manufactured by BAXTER, which are specifically designed for use in endoscopy, minimally invasive surgical procedures, or for applying the product to large or hard-to-reach surfaces. When using such devices, follow the instructions for their use carefully. For certain applications, biocompatible materials such as collagen patches can be used as a carrier or for mechanical reinforcement of the glue joints.
Application using a spray device
When applying the TISSEEL Lyo product using a spray device, the manufacturer's recommendations for pressure and distance from the tissue should be followed in accordance with the ranges given below:
| Recommended pressure and distance from tissue values and spray device for applying the TISSEEL Lyo product | |||||
| Surgical treatment | Spray set to be used | Applicator tips to be used | Pressure regulator to be used | Recommended distance from target tissue | Recommended spray pressure |
| Open wound | Tisseel/Artiss spray set | nd. | EasySpray | 10–15 cm | 1.5–2.0 bar (21.5–28.5 psi) |
| Tisseel/Artiss spray set — package of 10 | nd. | EasySpray | |||
| Laparoscopic/minimally invasive procedures | nd. | Duplospray MIS applicator 20 cm | Duplospray MIS regulator 1.5 bar | 2–5 cm | 1.2–1.5 bar (18–22 psi) |
| Duplospray MIS applicator 30 cm | |||||
| Duplospray MIS applicator 40 cm | |||||
| Spray Set 360 applicator with endoscopic Snap Lock | |||||
| Spray Set 360 applicator with endoscopic securing strap | |||||
| Exchangeable tip | |||||
When spraying the TISSEEL Lyo product, it is necessary to monitor changes in arterial pressure, pulse, arterial oxygen saturation, and end-expiratory CO2 concentration due to the possibility of air or gas embolism (see section 2).
In the case of administering the TISSEEL Lyo product in a closed chest or abdominal cavity, the use of the DuploSpray MIS applicator and control system is recommended. Further information can be found in the DuploSpray MIS device instruction manual.
When administering the TISSEEL Lyo product in a closed chest or abdominal cavity, it is recommended to use the DuploSpray MIS applicator and control system. For more information, refer to the DuploSpray MIS device instruction manual.
Removal of residues
Any unused remains of the medicinal product or its waste should be disposed of in accordance with local regulations.
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterTakeda Manufacturing Austria AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tisseel LioDosage form: Solution, 100 mg/mlActive substance: acetylcysteineManufacturer: Lek Pharmaceuticals d.d.Prescription requiredDosage form: Solution, 10 mg/mlActive substance: calcium folinateManufacturer: Fresenius Kabi Austria GmbHPrescription requiredDosage form: Solution, 10 mg/mlActive substance: calcium folinatePrescription required
Online doctors for Tisseel Lio
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tisseel Lio – subject to medical assessment and local rules.














