
Tisercin
Ask a doctor about a prescription for Tisercin

How to use Tisercin
Leaflet attached to the packaging: information for the user
Tisercin
25 mg/ml, solution for injection
Levomepromazine
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Tisercin and what is it used for
- 2. Important information before using Tisercin
- 3. How to use Tisercin
- 4. Possible side effects
- 5. How to store Tisercin
- 6. Contents of the packaging and other information
1. What is Tisercin and what is it used for
Tisercin is a medicine used to treat mental disorders (neuroleptic).
Indications:
Mental illnesses with motor and psychomotor agitation.
Paranoid syndromes (schizophrenia).
As an adjunctive treatment in certain accompanying symptoms of epilepsy, mental retardation,
in depression with anxiety.
Potentiating the analgesic effect of other medicines.
Preparation and deepening of general anesthesia.
2. Important information before using Tisercin
When not to use Tisercin:
- if the patient is allergic to levomepromazine or any of the other ingredients of this medicine (listed in section 6);
- if the patient is hypersensitive (allergic) to medicines with the same action (e.g. chlorpromazine, thioridazine, fluphenazine, pipotiazine, trifluperazine) or has experienced hypersensitivity to light during the use of such medicines,
- if the patient is taking medicines used to treat high blood pressure (hypotensive),
- if the patient is taking medicines from the group of MAO inhibitors (monoamine oxidase) used to treat e.g. depression (such as selegiline, moclobemide),
- if the patient is using significant amounts of substances that depress the central nervous system (alcohol, sleeping pills, narcotics),
- if the patient has narrow-angle glaucoma,
- if the patient has urinary retention problems,
- if the patient has Parkinson's disease,
- if the patient has multiple sclerosis (a disease of the central nervous system),
- if the patient has myasthenia (pathological muscle weakness) or hemiplegia (complete paralysis of the left or right side of the body),
- if the patient has severe kidney or liver dysfunction,
- if the patient has serious heart problems (cardiomyopathy, heart failure),
- if the patient has low blood pressure,
- if the patient has a blood system disorder,
- if the patient has porphyria metabolism disorders,
- if the patient is breastfeeding,
- the medicine is contraindicated in children under 12 years of age.
Warnings and precautions
Before starting treatment with Tisercin, discuss it with your doctor, pharmacist or nurse.
Tisercin in the form of injections should be administered under close medical supervision, therefore
immediately inform your doctor if any of the following situations apply to you.
- If the patient has a cardiovascular disease (hypotension, arrhythmia, circulation disorders) or a history of it, especially if the patient is elderly (over 65 years old); this allows you to avoid possible serious complications.
- If the patient has had a stroke or is at increased risk of stroke.
- If the patient has diabetes, or is at increased risk of developing diabetes, because Tisercin may increase blood sugar levels. For this reason, blood sugar levels should be monitored more frequently than usual during therapy.
- If the patient has epilepsy.
- If the patient has other central nervous system diseases, because Tisercin may affect their symptoms or the patient may be more sensitive to some side effects.
- If the patient has impaired liver and/or kidney function.
- If there have been blood clots in the patient's or their family's history, because similar medicines have been associated with the formation of blood clots.
- In case of hypersensitivity (swelling of the lips, mouth or throat causing difficulty swallowing or breathing, annoying skin itching) the use of the medicine should be stopped immediately.
- If the patient experiences unexplained fever, especially in combination with other symptoms such as stiffness and/or uncontrolled muscle contractions and symptoms from the autonomic nervous system (e.g. palpitations, irregular heartbeat, unstable blood pressure, sweating, rapid changes in basic life parameters, confusion) that may lead to coma, treatment should be stopped immediately. In case of fever, you should contact your doctor immediately.
- In elderly patients with dementia treated with antipsychotic medicines, the risk of death is slightly increased compared to patients not taking antipsychotic medicines.
Before starting treatment and during therapy, it is recommended to regularly monitor the following parameters:
- arterial blood pressure, especially if it is unstable or low
- liver function (especially in people with existing liver function disorders)
- blood morphology with a smear in case of fever, sore throat (at the beginning of treatment and during long-term use of the medicine)
- ECG (in people with heart and circulatory diseases and in elderly patients over 65 years old)
- potassium level in serum.
Children and adolescents
The medicine is contraindicated in children under 12 years of age.
Tisercin and other medicines
Tell your doctor and/or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Remember that possible interactions with other medicines also apply to medicines taken some time ago, as well as those that will be taken in the near future.
Before starting to take any medicine (especially one that acts on the central nervous system) during or within a month after stopping treatment with Tisercin, inform your doctor about this intention.
Tisercin should not be administered simultaneously with:
- medicines used to treat high blood pressure,
- certain antidepressants (MAO inhibitors).
The use of Tisercin with the following medicines requires consultation with a doctor:
- medicines that act on the central nervous system (including sedatives, sleeping pills, strong painkillers, general anesthetics, antidepressants, antiepileptics, amphetamines),
- levodopa, a medicine used in Parkinson's disease,
- antidiabetic medicines,
- certain medicines used to treat irregular heartbeat or heart disease, some antibiotics (macrolides), medicines used to treat fungal infections, medicines used in allergies (antihistamines), cisapride, some medicines that increase urine production and excretion,
- dilevalol
- medicines that increase skin sensitivity to sunlight
- deferoksamina - used to remove excess iron or aluminum from the body,
- epinephrine (adrenaline), used to treat allergies.
In cases where Tisercin is used in combination with any of these medicines, close medical supervision is recommended, and in justified cases - modification of the dosage.
Others
- vitamin C should be administered to reduce the vitamin deficiency caused by Tisercin.
Tisercin and alcohol
Consuming alcoholic beverages is strictly prohibited during treatment with Tisercin, as well as for 4-5 days after stopping the medicine.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, suspects she may be pregnant or plans to have a child, she should consult a doctor or pharmacist before using this medicine.
In newborns whose mothers used Tisercin in the last trimester (the last three months of pregnancy), the following may occur: tremors, increased and/or decreased muscle tone, sleepiness, agitation, breathing disorders and feeding disorders. If these symptoms occur in your child, you should contact your doctor.
During pregnancy, the use of the medicine should be limited to exceptional cases and only when the doctor considers that the potential benefits outweigh the possible risk.
The active substance of the medicine passes into breast milk. Due to the lack of relevant clinical data, the use of this medicine is contraindicated during breastfeeding.
Tisercin may affect fertility in women and men.
Driving and operating machines
At the beginning of treatment - for an individually determined period - you should refrain from driving vehicles and operating machines. Later, these restrictions may be maintained or lifted, depending on the result of the consultation with the doctor.
Tisercin contains sodium
This medicine contains approximately 2.3 mg of sodium, less than 1 mmol (23 mg) per dose (1 ml), which means the medicine is considered "sodium-free". If the doctor uses a saline solution to dilute Tisercin, the amount of sodium from the diluent should also be taken into account.
3. How to use Tisercin
This medicine should always be used in accordance with the doctor's recommendations. In case of doubts, consult a doctor or pharmacist.
Follow the doctor's instructions regarding the dose of the medicine, the method of administration and the duration of treatment.
Tisercin is administered intramuscularly or intravenously (only in slow infusion).
Parenteral administration is used when it is impossible to administer the medicine orally. The daily dose is usually 75-100 mg (in 2-3 divided doses), provided the patient is bedridden and their vital functions are closely monitored.
When administered intramuscularly, the medicine will be given in a deep intramuscular injection.
When administered intravenously, the medicine will be used only in the form of a diluted solution in slow drip infusion (50-100 mg of Tisercin in 250 ml of physiological saline or glucose solution).
To avoid excessive drops in blood pressure when changing position to standing, it is recommended to lie down for at least half an hour after administering the first dose. If the patient experiences dizziness after administering the medicine, it is recommended to lie down after each dose of the medicine.
Elderly patients (over 65 years old) are more sensitive to the side effects of Tisercin, therefore in this group of patients it is recommended to start treatment with small doses and gradually increase them.
Using a higher dose of Tisercin than recommended
In case of overdose, the following may occur: hypotension, fever, conduction disorder, arrhythmia, which may lead to sudden death or cardiac arrest, muscle stiffness, muscle contractions, sleepiness, coma, central nervous system stimulation (epileptic seizures) and malignant neuroleptic syndrome - a dangerous reaction characterized by fever, muscle stiffness, confusion, sweating and changes in heart rhythm.
Overdose of Tisercin, especially in combination with alcohol or other medicines that act on the central nervous system, may lead to death.
Stopping the use of Tisercin
Without the doctor's recommendation, you should not stop taking Tisercin injections, even if you feel better, unless severe side effects have occurred.
The doctor may recommend stopping treatment by gradually reducing the dose of the medicine, as sudden withdrawal of the medicine may lead to a relapse of psychotic symptoms, anxiety, increased anxiety, insomnia, nausea, vomiting, headaches, tremors, sweating and tachycardia.
In case of any further doubts related to the use of this medicine, consult a doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects are usually mild, disappear during treatment and do not require discontinuation of treatment.
There is a lack of relevant data on the frequency of occurrence.
The following side effects may occur:
- The most important and most common side effect is a drop in blood pressure when changing body position to standing, which leads to dizziness, weakness or fainting.
- Pancytopenia (decrease in the number of all morphotic elements of blood), agranulocytosis (significant decrease in the number of white blood cells, leading to increased susceptibility to infections), leukopenia (decrease in the number of white blood cells), thrombocytopenia (decrease in the number of platelets), eosinophilia (increase in the number of blood cells called eosinophils), increased erythrocyte sedimentation rate.
- Pseudoanaphylactic reactions (dangerous allergic reaction causing difficulty breathing or dizziness), throat swelling, swelling of the ankles, feet and toes, asthma attack.
- Weight loss, vitamin deficiency.
- Glucose intolerance, high blood sugar levels.
- Low sodium levels in the blood, inappropriate secretion of antidiuretic hormone (with symptoms such as concentrated urine, feeling of thirst, nausea, muscle cramps, confusion and seizures).
- Relapse of psychotic symptoms, catatonia (movement disorder), confusion, disorientation, visual hallucinations, slurred speech, sleepiness.
- Seizures, increased intracranial pressure, extrapyramidal symptoms (muscle stiffness, muscle contractions and involuntary movements), withdrawal symptoms.
- Deposits in the lens and cornea, pigmentary retinopathy (eye disease related to the deposition of pigment in the retina).
- Abnormal ECG recording, irregular heartbeat, rapid heartbeat, atrial fibrillation, cardiac arrest, sudden death (unexplained or cardiac).
- Blood clots in the veins, especially in the legs (symptoms include swelling, pain, redness of the leg); blood clots can move through the blood vessels to the lungs, causing chest pain and difficulty breathing. If you notice any of these symptoms, you should immediately consult a doctor.
- Ischemic colitis (abdominal pain, bloody diarrhea), vomiting, nausea, constipation, abdominal discomfort, nausea, dry mouth.
- Liver damage (jaundice, bile stasis).
- Severe skin inflammation, hives, rash, increased skin sensitivity to sunlight, skin pigmentation.
- Difficulty urinating, change in urine color.
- Withdrawal syndrome in newborns
- Lactation, menstrual disorders, very rarely abnormal uterine contractions.
- Painful and prolonged erection (priapism).
- Malignant neuroleptic syndrome (a serious reaction to the medicine characterized by high fever, muscle stiffness, confusion, sweating and changes in heart rhythm), elevated body temperature (unexplained fever).
- In the case of long-term use of medicines such as Tisercin, mild (non-cancerous) tumors of the pituitary gland may occur.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should inform your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products Al.
Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help gather more information on the safety of the medicine.
5. How to store Tisercin
The medicine should be stored at a temperature below 25°C. Store in the original packaging to protect from light.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date (EXP) stated on the packaging. The expiry date refers to the last day of the specified month.
Do not use this medicine if you notice visible signs of spoilage (e.g. discoloration).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Tisercin contains
The active substance of the medicine is levomepromazine. Each 1 ml solution for injection contains 25 mg of levomepromazine.
The other ingredients are: monothioglycerol, anhydrous citric acid, sodium chloride, water for injections.
What Tisercin looks like and what the packaging contains
Completely transparent, odorless liquid.
Type B ampoules with a break point of the OPC (one-point cut) type, packaged in PVC/PET/PE blisters and a cardboard box.
Each packaging contains 10 ampoules (2 blisters of 5 ampoules).
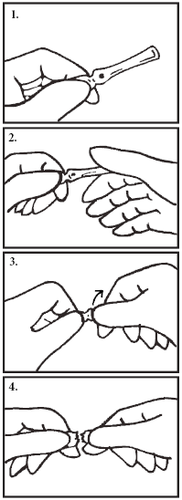
How to open the ampoule (for right-handed users):
Hold the ampoule body between the thumb and bent index finger of the left hand. The ampoule is held so that the painted break point is at the top (Figure 1).
Grasp the ampoule head with the thumb and bent index finger of the other (right) hand.
The thumb must cover the break point on the ampoule (Figure 2).
Press the right thumb and the opposing left index finger and perform a moderate and steady bending motion without moving the hands away from or towards each other (Figure 3).
The ampoule neck may break at any time after starting to press and you may not feel when the ampoule breaks (Figure 4).
Marketing authorization holder
EGIS PHARMACEUTICALS PLC
Keresztúri út 30-38
1106 Budapest.
HUNGARY
Manufacturer
EGIS Pharmaceuticals PLC
Bökényföldi ut 118-120,
1165 Budapest
HUNGARY
To obtain more detailed information, please contact the representative of the marketing authorization holder in Poland.
EGIS Polska Sp. z o.o.
ul. Komitetu Obrony Robotników 45 D
02-146 Warsaw
Phone: +48 22 417 92 00
Date of last update of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterEGIS Pharmaceuticals PLC
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TisercinDosage form: Tablets, 25 mgActive substance: levomepromazinePrescription requiredDosage form: Tablets, 25 mgActive substance: levomepromazineManufacturer: Egis Pharmaceuticals PLCPrescription requiredDosage form: Solution, 25 mg/mlActive substance: chlorpromazineManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription required
Alternatives to Tisercin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tisercin in Ukraina
Alternative to Tisercin in Hiszpania
Online doctors for Tisercin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tisercin – subject to medical assessment and local rules.









