
Ticagrelor Holsten

Ask a doctor about a prescription for Ticagrelor Holsten

How to use Ticagrelor Holsten
CHARACTERISTICS OF THE MEDICINAL PRODUCT
1. NAME OF THE MEDICINAL PRODUCT
Ticagrelor Holsten, 60 mg, film-coated tablets
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each film-coated tablet contains 60 mg of ticagrelor.
The full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Film-coated tablet.
Round (8.1 x 8.1 mm), biconvex, pink tablets marked with the number "60" on one side and smooth on the other side.
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Ticagrelor Holsten, in combination with acetylsalicylic acid (ASA), is indicated to prevent atherothrombotic events in adult patients:
- with acute coronary syndrome (ACS) or
- with a history of myocardial infarction (MI) and a high risk of atherothrombotic events (see sections 4.2 and 5.1).
4.2 Posology and method of administration
Dosage
Patients taking Ticagrelor Holsten should also take a daily maintenance dose of ASA 75-150 mg, unless individually contraindicated.
Acute coronary syndromes
Treatment with Ticagrelor Holsten should be initiated with a loading dose of 180 mg (2 tablets of 90 mg) and continued at a dose of 90 mg twice daily.
In patients with ACS, the duration of treatment with Ticagrelor Holsten 90 mg twice daily should be at least 12 months, unless there are clinical reasons to discontinue the treatment (see section 5.1).
Discontinuation of ASA can be considered after 3 months in patients with ACS who have undergone percutaneous coronary intervention (PCI), and who have a high risk of bleeding. In such cases, administration of ticagrelor as the only antiplatelet drug should be continued for 9 months (see section 4.4).
History of myocardial infarction
The recommended dose of Ticagrelor Holsten is 60 mg twice daily, if prolonged treatment is needed for patients with a history of MI at least one year prior and at high risk of atherothrombotic events (see section 5.1). Treatment can be started without interruption as a continuation of the initial 12-month treatment with Ticagrelor Holsten 90 mg or another ADP receptor inhibitor in patients with ACS and high risk of atherothrombotic events. Treatment can also be initiated up to 2 years after MI or within one year after discontinuation of a previous ADP receptor inhibitor. Data on the efficacy and safety of ticagrelor beyond 3 years of long-term treatment are limited.
If a switch from another antiplatelet agent is needed, the first dose of Ticagrelor Holsten should be administered 24 hours after the last dose of the other antiplatelet agent.
Missed dose
Patients should also be advised to avoid dosing errors. If a dose of Ticagrelor Holsten is missed, the patient should take only the next dose at the scheduled time, as prescribed.
Special populations
Elderly patients
No dose adjustment is required in elderly patients (see section 5.2).
Renal impairment
No dose adjustment is required in patients with renal impairment (see section 5.2).
Hepatic impairment
Ticagrelor has not been studied in patients with severe hepatic impairment and therefore its use is contraindicated in such patients (see sections 4.2, 4.4 and 5.2). There is limited experience with ticagrelor in patients with moderate hepatic impairment. No dose adjustment is required, but ticagrelor should be used with caution (see sections 4.4 and 5.2). In patients with mild hepatic impairment, no dose adjustment is required (see section 5.2).
Paediatric population
The safety and efficacy of ticagrelor in children below 18 years of age have not been established.
Ticagrelor is not indicated in children for the treatment of sickle cell disease (see sections 5.1 and 5.2).
Method of administration
Oral use.
Ticagrelor Holsten can be administered with or without food.
In patients who have difficulty swallowing the tablet(s) whole, the tablets can be crushed to a fine powder, mixed with half a glass of water, and administered immediately. The glass should then be rinsed with water (an additional half glass, up to a maximum of 125 ml) and the contents swallowed.
The crushed tablet(s) mixed with water can also be administered via a nasogastric tube (CH8 or larger). It is important to rinse the nasogastric tube with water (at least 50 ml, up to a maximum of 125 ml) after administration of the mixture.
4.3 Contraindications
- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 (see section 4.8).
- 6.1 (see section 4.8).
- Active pathological bleeding.
- History of intracranial haemorrhage (see section 4.8).
- Severe hepatic impairment (see sections 4.2, 4.4 and 5.2).
- Concomitant use of ticagrelor and strong CYP3A4 inhibitors (e.g. ketoconazole, clarithromycin, nefazodone, ritonavir and atazanavir), as this may lead to a significant increase in exposure to ticagrelor (see section 4.5).
4.4 Special warnings and precautions for use
Bleeding risk
In patients at increased risk of bleeding, the benefit of treatment with Ticagrelor Holsten should be weighed against the risk of bleeding (see sections 4.8 and 5.1). In cases where there are clinical reasons to use ticagrelor, it should be used with caution in the following patient groups:
- Patient with a history of bleeding (e.g. recent trauma, surgical procedures, bleeding diathesis, active or recent gastrointestinal bleeding) or at increased risk of bleeding. Ticagrelor is contraindicated in patients with active pathological bleeding, a history of intracranial haemorrhage, or severe hepatic impairment (see section 4.3).
- Patient taking concomitantly medications that may increase the risk of bleeding (e.g. non-steroidal anti-inflammatory drugs (NSAIDs), oral anticoagulants and/or fibrinolytic agents) within 24 hours prior to ticagrelor administration.
In the two randomised, double-blind, controlled trials (TICO and TWILIGHT) in patients with ACS who underwent PCI with a drug-eluting stent, discontinuation of ASA after 3 months of dual antiplatelet therapy with ticagrelor and ASA, and continuation of ticagrelor as the only antiplatelet agent for 9 and 12 months, respectively, reduced the risk of bleeding and did not result in an increased risk of observed major adverse cardiac events (MACE) compared to continued dual antiplatelet therapy. The decision to discontinue ASA after 3 months and continue ticagrelor as the only antiplatelet agent for 9 months in patients at high risk of bleeding should be made on an individual basis, taking into account the risk of bleeding versus the risk of thrombotic events (see section 4.2).
Platelet transfusion did not reverse the antiplatelet effect of ticagrelor in healthy volunteers and is unlikely to be clinically useful in patients with bleeding.
Desmopressin has not been shown to shorten the bleeding time in patients on ticagrelor (see section 4.5).
Fibrinolytic therapy (aminocaproic acid or tranexamic acid) and/or recombinant factor VIIa may increase haemostasis. Ticagrelor may be re-administered when the cause of bleeding has been identified and controlled.
Surgical procedures
Patient should be instructed to inform physicians and dentists that they are taking ticagrelor before any surgery is scheduled and before any new drug is taken.
In the PLATO study, among patients who underwent coronary artery bypass grafting (CABG), more bleeding was observed in the ticagrelor group than in the clopidogrel group if the treatment was not discontinued before surgery, but if the treatment was discontinued at least 2 days before surgery, the number of major bleedings was similar in both groups (see section 4.8). If a patient is to undergo planned surgical procedure, ticagrelor should be discontinued at least 5 days before surgery (see section 5.1).
Patient with a history of ischaemic stroke
Patient with ACS and a history of ischaemic stroke may be treated with ticagrelor for up to 12 months (PLATO study).
Patient with a history of myocardial infarction and ischaemic stroke were not included in the PEGASUS study. Therefore, due to lack of data, it is not recommended to treat these patients for more than one year.
Hepatic impairment
Concomitant use of ticagrelor in patients with severe hepatic impairment is contraindicated (see sections 4.2 and 4.3). There is limited experience with ticagrelor in patients with moderate hepatic impairment, and therefore caution should be exercised in these patients (see sections 4.2 and 5.2).
Patient at risk of bradyarrhythmias
Monitoring of Holter ECG recordings revealed an increased frequency of ventricular pauses during ticagrelor treatment compared to clopidogrel. Patients at increased risk of bradyarrhythmias (e.g. patients without a pacemaker with sick sinus syndrome, 2nd or 3rd degree atrioventricular block, or with bradycardia-related syncope) were excluded from the main studies assessing the safety and efficacy of ticagrelor. Therefore, due to limited clinical experience, ticagrelor should be used with caution in this patient population (see section 5.1).
Caution should also be exercised when ticagrelor is co-administered with medicinal products that may induce bradyarrhythmias. However, there was no evidence of clinically significant adverse effects observed in the PLATO study after concomitant administration with one or more medicinal products that induce bradyarrhythmias (i.e. 96% beta-blockers, 33% calcium channel blockers diltiazem and verapamil, and 4% digoxin) (see section 4.5).
In the PLATO study, in a Holter substudy, patients on ticagrelor had more ventricular pauses >3 seconds than patients on clopidogrel in the acute phase of ACS. The increase in ventricular pauses during ticagrelor treatment was more pronounced in patients with chronic heart failure than in the overall ACS population, but not in the one-month follow-up of ticagrelor treatment, or compared to clopidogrel. No clinically significant adverse consequences were associated with this imbalance (including syncope or pacemaker implantations) in this patient population (see section 5.1).
Post-marketing experience has reported cases of bradyarrhythmias and atrioventricular (AV) blocks (see section 4.8), mainly in patients with ACS, where myocardial ischaemia and concomitantly administered medications that lower heart rate or affect conduction in the heart are potential confounding factors. Before adjusting the treatment, the clinical condition of the patient and concomitantly administered medications should be assessed as potential causes.
Dyspnoea
Patient treated with ticagrelor have reported dyspnoea. Dyspnoea is usually mild to moderate and often resolves without the need to discontinue the medicinal product. In patients with asthma/chronic obstructive pulmonary disease (COPD), there may be an increased absolute risk of dyspnoea when treated with ticagrelor. Ticagrelor should be used with caution in patients with a history of asthma and/or COPD. The mechanism of dyspnoea is unknown. If a patient reports new, worsening, or persistent dyspnoea, a thorough investigation should be performed and if the patient is not tolerating the condition, the treatment with ticagrelor should be discontinued. Further information is provided in section 4.8.
Central sleep apnoea
Post-marketing experience has reported cases of central sleep apnoea, including Cheyne-Stokes respiration. If central sleep apnoea is suspected, further clinical evaluation should be considered.
Increased creatinine levels
During treatment with ticagrelor, an increase in creatinine levels may occur. The mechanism of this phenomenon has not been established. Renal function should be monitored according to clinical practice. In patients with ACS, it is recommended to monitor renal function also after one month of treatment with ticagrelor, with particular attention to patients aged ≥75 years, patients with moderate to severe renal impairment, and those taking angiotensin receptor blockers (ARBs).
Increased uric acid levels
During treatment with ticagrelor, hyperuricaemia may develop (see section 4.8). Caution should be exercised in patients with hyperuricaemia or a history of gout.
As a precautionary measure, the use of ticagrelor is not recommended in patients with uric acid nephropathy.
Thrombotic thrombocytopenic purpura (TTP)
Very rare cases of TTP have been reported during treatment with ticagrelor. TTP is characterised by thrombocytopenia and microangiopathic haemolytic anaemia associated with neurological symptoms, renal dysfunction, or fever. TTP is a potentially life-threatening condition requiring prompt treatment, including plasmapheresis.
Interference with tests for heparin-induced thrombocytopenia (HIT)
In the functional assay for heparin-induced platelet activation (HIPA) used for the diagnosis of HIT, patient antibodies against the platelet factor 4/heparin complex activate platelets from healthy donors in the presence of heparin.
False negative results for functional platelet assays (including HIPA) used for the diagnosis of HIT have been reported in patients taking ticagrelor. This is due to inhibition of the P2Y12 receptor on donor platelets by ticagrelor present in the patient's serum/plasma. Information on concomitant treatment with ticagrelor is required for the interpretation of functional platelet assay results used for the diagnosis of HIT.
In patients who develop HIT, the benefit/risk ratio of continuing ticagrelor should be assessed, taking into account both the prothrombotic state of HIT and the increased risk of bleeding during concomitant treatment with anticoagulant and ticagrelor.
Other
Based on the observed relationship between the maintenance dose of ASA and the relative efficacy of ticagrelor compared to clopidogrel in the PLATO study, concomitant use of ticagrelor and high maintenance doses of ASA (>300 mg) is not recommended (see section 5.1).
Premature discontinuation of treatment
Premature discontinuation of any antiplatelet therapy, including Ticagrelor Holsten, may result in an increased risk of cardiac events, myocardial infarction, or stroke due to the underlying disease. Therefore, premature discontinuation of treatment should be avoided.
Sodium
Ticagrelor Holsten contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
4.5 Interaction with other medicinal products and other forms of interaction
Ticagrelor is primarily metabolised by the cytochrome P450 3A4 enzyme and is also a mild inhibitor of this enzyme.
Ticagrelor is also a substrate of the P-glycoprotein (P-gp) and a weak inhibitor of P-gp and may increase exposure to P-gp substrates.
Effects of other medicinal products on ticagrelor
CYP3A4 inhibitors
- Strong CYP3A4 inhibitors - concomitant use of ketoconazole with ticagrelor resulted in a 2.4-fold increase in C and a 7.3-fold increase in AUC of ticagrelor. C and AUC of the active metabolite were decreased by 89% and 56%, respectively. It is anticipated that other strong CYP3A4 inhibitors (clarithromycin, nefazodone, ritonavir, and atazanavir) will have a similar effect and therefore concomitant use of strong CYP3A4 inhibitors with ticagrelor is contraindicated (see section 4.3).
- Moderate CYP3A4 inhibitors - concomitant use of diltiazem and ticagrelor resulted in a 69% increase in C of ticagrelor and a 2.7-fold increase in AUC, and a 38% decrease in C of the active metabolite, with no effect on its AUC. Ticagrelor did not affect the plasma exposure of diltiazem. Other moderate CYP3A4 inhibitors (e.g. amprenavir, aprepitant, erythromycin, and fluconazole) may have a similar effect and can be co-administered with ticagrelor.
- A 2-fold increase in exposure to ticagrelor was observed when ticagrelor was co-administered with large amounts of grapefruit juice (3 x 200 ml). It is not expected that this increase in exposure would be clinically significant for most patients.
CYP3A4 inducers
Concomitant use of rifampicin and ticagrelor decreased C and AUC of ticagrelor by 73% and 86%, respectively. C of the active metabolite was unchanged, while its AUC was decreased by 46%. It is anticipated that other CYP3A4 inducers (e.g. phenytoin, carbamazepine, and phenobarbital) will also decrease exposure to ticagrelor. Concomitant use of ticagrelor and strong CYP3A4 inducers may decrease the efficacy of ticagrelor and is not recommended.
Cyclosporin (P-gp and CYP3A inhibitor)
Concomitant use of cyclosporin (600 mg) and ticagrelor increased C of ticagrelor by 2.3-fold and AUC by 2.8-fold. In the presence of cyclosporin, AUC of the active metabolite of ticagrelor increased by 32% and C decreased by 15%.
There are no data on the concomitant use of ticagrelor with other potent inhibitors of P-gp and moderate inhibitors of CYP3A4 (e.g. verapamil, quinidine), which may increase exposure to ticagrelor. If co-administration cannot be avoided, it should be done with caution.
Other
Clinical interaction studies have shown that concomitant use of ticagrelor with heparin, enoxaparin, or ASA, or desmopressin did not affect the pharmacokinetics of ticagrelor or its active metabolite, or ADP-induced platelet aggregation compared to ticagrelor alone. If clinically indicated, medicinal products that affect haemostasis should be used with caution in combination with ticagrelor.
In patients with ACS treated with morphine, a delay and a decrease in exposure to oral P2Y12 inhibitors, including ticagrelor and its active metabolite (a 35% decrease in exposure to ticagrelor), was observed. The clinical significance of this interaction is unknown, but data suggest a potential reduction in the efficacy of ticagrelor in patients receiving ticagrelor and morphine concomitantly.
In patients with ACS, where rapid P2Y12 inhibition is critical, an alternative antiplatelet agent may be considered.
Effects of ticagrelor on other medicinal products
Medicinal products metabolised by CYP3A4
- Simvastatin - concomitant use of ticagrelor with simvastatin resulted in an 81% increase in C of simvastatin and a 56% increase in AUC, and a 64% increase in C of simvastatin acid and a 52% increase in its AUC, with single cases of 2- or 3-fold increases. Concomitant use of ticagrelor and simvastatin at a dose greater than 40 mg daily may increase the risk of simvastatin-related adverse effects and should be considered in the assessment of the potential benefits of this combination. No effect of simvastatin on the plasma exposure of ticagrelor was observed. Ticagrelor may have a similar effect on the use of lowastatin. Concomitant use of ticagrelor and simvastatin or lowastatin at doses greater than 40 mg is not recommended.
- Atorvastatin - concomitant use of atorvastatin and ticagrelor resulted in a 23% increase in C of atorvastatin acid and a 36% increase in AUC, and similar increases in AUC and C of all metabolites of atorvastatin acid. It is considered that this is not clinically significant.
- It cannot be excluded that a similar effect may occur with other statins metabolised by CYP3A4. In the PLATO study, patients were treated with various statins and in 93% of all patients, there were no concerns regarding safety related to the use of statins.
Ticagrelor is a moderate inhibitor of CYP3A4. Concomitant use of ticagrelor and CYP3A4 substrates with a narrow therapeutic index (e.g. cisapride and ergot alkaloids) is not recommended, as ticagrelor may increase exposure to these medicinal products.
P-gp substrates (including digoxin, cyclosporin)
Concomitant use of ticagrelor with digoxin increased C and AUC of digoxin by 75% and 28%, respectively. Mean minimum digoxin concentrations increased by approximately 30% when co-administered with ticagrelor, with single maximum concentrations increasing to 2-fold. The presence of digoxin did not affect C and AUC of ticagrelor and its active metabolite. Therefore, appropriate clinical monitoring and/or laboratory monitoring is recommended when medicinal products with a narrow therapeutic index that are P-gp dependent, such as digoxin and ticagrelor, are co-administered.
Ticagrelor did not affect the plasma exposure of cyclosporin. The effect of ticagrelor on other P-gp substrates has not been studied.
Medicinal products metabolised by CYP2C9
Concomitant use of ticagrelor and tolbutamide did not affect the plasma exposure of either medicinal product, suggesting that ticagrelor is not an inhibitor of CYP2C9 and is unlikely to affect the metabolism of medicinal products such as warfarin or tolbutamide via the CYP2C9 pathway.
Rosuvastatin
Ticagrelor may affect the renal excretion of rosuvastatin, increasing the risk of rosuvastatin accumulation. Although the exact mechanism is unknown, concomitant use of ticagrelor and rosuvastatin has led to worsening of renal function, increased CPK (creatine phosphokinase) activity, and rhabdomyolysis in some cases.
Oral contraceptives
Concomitant use of ticagrelor with levonorgestrel and ethinyl estradiol resulted in approximately a 20% increase in exposure to ethinyl estradiol, but did not affect the pharmacokinetics of levonorgestrel. It is not anticipated that there will be a clinically significant effect on the efficacy of oral contraceptives when levonorgestrel and ethinyl estradiol are co-administered with ticagrelor.
Medicinal products that induce bradyarrhythmias
Given the observed, usually asymptomatic, ventricular pauses and bradycardia, caution should be exercised when ticagrelor is co-administered with medicinal products that induce bradyarrhythmias (see section 4.4). In the PLATO study, however, there was no evidence of clinically significant adverse effects observed after concomitant administration with one or more medicinal products that induce bradyarrhythmias (i.e. 96% beta-blockers, 33% calcium channel blockers: diltiazem and verapamil, and 4% digoxin).
Concomitant use with other medicinal products
In clinical studies, ticagrelor has been co-administered with ASA, proton pump inhibitors, statins, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers, for long periods of time due to the need to treat co-morbid conditions, as well as with heparin, low molecular weight heparin, and intravenous GP IIb/IIIa inhibitors for short periods of time (see section 5.1). No significant clinically relevant interactions have been observed with these medicinal products.
Concomitant use of ticagrelor with heparin, enoxaparin, or desmopressin did not affect the activated partial thromboplastin time (aPTT), activated clotting time (ACT), or factor Xa activity. However, due to potential pharmacodynamic interactions, caution should be exercised when ticagrelor is co-administered with medicinal products that affect haemostasis.
Given the observed bleeding with selective serotonin reuptake inhibitors (SSRIs) (e.g. paroxetine, sertraline, and citalopram), caution should be exercised when SSRIs are used concomitantly with ticagrelor, as this may increase the risk of bleeding.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Women of childbearing potential should use appropriate contraceptive methods to prevent pregnancy while taking ticagrelor.
Pregnancy
There are no or limited amount of data from the use of ticagrelor in pregnant women. Animal studies have shown reproductive toxicity (see section 5.3). Ticagrelor is not recommended during pregnancy.
Breast-feeding
Available pharmacodynamic/toxicological data in animals have shown excretion of ticagrelor and its active metabolites in milk (see section 5.3). A risk to the breast-fed child cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from ticagrelor therapy, taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Fertility
In animal studies, ticagrelor did not affect fertility in male and female rats (see section 5.3).
4.7 Effects on ability to drive and use machines
Ticagrelor has no or negligible influence on the ability to drive and use machines. Dizziness and confusion have been reported in patients treated with ticagrelor. Therefore, patients who experience these symptoms should be cautious whilst driving or using machines.
4.8 Undesirable effects
Summary of the safety profile
The safety profile of ticagrelor was evaluated in two large phase 3 studies (PLATO and PEGASUS), involving over 39,000 patients (see section 5.1).
In the PLATO study, more patients in the ticagrelor group than in the clopidogrel group discontinued the study medication due to adverse events (7.4% versus 5.4%).
In the PEGASUS study, more patients in the ticagrelor group than in the ASA monotherapy group discontinued the study medication due to adverse events (16.1% in the ticagrelor 60 mg twice daily plus ASA group versus 8.5% in the ASA monotherapy group). The most common adverse reactions in patients taking ticagrelor were bleeding and dyspnoea (see section 4.4).
Tabular list of adverse reactions
The following adverse reactions were identified from clinical trials or post-marketing experience with ticagrelor (Table 1).
Adverse reactions are ranked by frequency, using the following convention: very common (≥1/10), common (≥1/100 to <1>Table 1 - Adverse reactions listed by frequency and System Organ Class (SOC)
| System Organ Class | Very common | Common | Uncommon | Frequency not known |
| Benign, malignant and unspecified neoplasms (including cysts and polyps) | Bleeding from tumoursa | |||
| Blood and lymphatic system disorders | Bleedingb | Thrombotic thrombocytopenic purpurac | ||
| Immune system disorders | Hypersensitivity, including anaphylactic reactionsc | |||
| Metabolism and nutrition disorders | Hyperuricaemiad | Gout/Gouty arthritise | ||
| Psychiatric disorders | Confusion | |||
| Nervous system disorders | Dizziness, syncope, headache | Intracranial haemorrhagef | ||
| Eye disorders | Eye bleedingg | |||
| Ear and labyrinth disorders | Vertigo of labyrinthine origin | Ear bleeding | ||
| Cardiac disorders | Bradyarrhythmia, AV blockh |
| System Organ Class | Very common | Common | Uncommon | Frequency not known |
| Vascular disorders | Hypotension | |||
| Respiratory, thoracic and mediastinal disorders | Dyspnoea | Bleeding in the respiratory tracti | ||
| Gastrointestinal disorders | Gastrointestinal bleeding, diarrhoea, nausea, vomiting, constipation | Intra-abdominal haemorrhagej | ||
| Skin and subcutaneous tissue disorders | Subcutaneous or skin bleedingk, rash, pruritus | |||
| Musculoskeletal and connective tissue disorders | Muscle bleeding | |||
| Renal and urinary disorders | Urinary tract bleeding | |||
| Reproductive system and breast disorders | Bleeding in the genital tractl | |||
| Investigations | Increased creatinine levels in the bloodm | |||
| Injury, poisoning and procedural complications | Post-procedural bleeding, post-traumatic bleeding |
Description of selected adverse reactions
Bleeding
PLATO study bleeding results
The overall outcome of bleeding in the PLATO study is presented in Table 2.
Table 2 - Analysis of all bleeding events, estimated using the Kaplan-Meier method at 12 months (PLATO)
| Ticagrelor 90 mg twice daily N=9235 | Clopidogrel N=9186 | p-value* | |
| PLATO-defined major bleeding | 11.6 | 11.2 | 0.4336 |
| PLATO-defined major bleeding that was fatal or life-threatening | 5.8 | 5.8 | 0.6988 |
| PLATO-defined major bleeding not related to CABG | 4.5 | 3.8 | 0.0264 |
| PLATO-defined major bleeding not related to procedures | 3.1 | 2.3 | 0.0058 |
| PLATO-defined major + minor bleeding | 16.1 | 14.6 | 0.0084 |
| PLATO-defined major + minor bleeding not related to procedures | 5.9 | 4.3 | <0.0001 |
| TIMI major bleeding | 7.9 | 7.7 | 0.5669 |
| TIMI major + minor bleeding | 11.4 | 10.9 | 0.3272 |
Ticagrelor and clopidogrel did not differ in the rate of PLATO-defined major bleeding that was fatal or life-threatening, PLATO-defined major bleeding overall, TIMI major bleeding, or TIMI major + minor bleeding. However, more PLATO-defined major + minor bleeding overall and PLATO-defined major + minor bleeding not related to procedures occurred in the ticagrelor group compared to the clopidogrel group (Table 2).
Bleeding related to CABG:
In the PLATO study, among the 42% of 1584 patients (12% of the cohort) who underwent CABG, 42% of patients experienced a PLATO-defined major bleeding that was fatal or life-threatening, with no difference observed between the treatment groups. Fatal bleeding after CABG occurred in 6 patients in each treatment group (see section 4.4).
Bleeding not related to CABG and bleeding not related to procedures:
Ticagrelor and clopidogrel did not differ in the rate of PLATO-defined major bleeding not related to CABG that was fatal or life-threatening. However, PLATO-defined major bleeding overall, TIMI major bleeding, and TIMI major + minor bleeding occurred more frequently in the ticagrelor group. Similarly, when all procedure-related bleeding was eliminated, more bleeding occurred in the ticagrelor group than in the clopidogrel group (Table 2). Discontinuation of study medication due to bleeding not related to procedures occurred more frequently in the ticagrelor group (2.9%) than in the clopidogrel group (1.2%; p<0.001).
Cerebral bleeding:
In the PLATO study, more non-procedure-related intracranial bleeding was observed with ticagrelor (n=27 bleeding events in 26 patients, 0.3%) than with clopidogrel (n=14 bleeding events, 0.2%), including 11 bleeding events in the ticagrelor group and 1 in the clopidogrel group that were fatal. No difference was observed in the overall rate of fatal bleeding.
PEGASUS study bleeding results
The overall outcome of bleeding in the PEGASUS study is presented in Table 3.
4.9 Overdose
Ticagrelor is well tolerated at a single dose of up to 900 mg. In a study with a single rising dose, gastrointestinal adverse events were dose-dependent. Other clinically significant adverse events that may occur with overdose include dyspnea and ventricular pauses (see section 4.8).
In the event of overdose, the above potential adverse events may occur, and monitoring of the electrocardiogram (ECG) should be considered.
Currently, there is no known antidote to reverse the effects of ticagrelor, and ticagrelor is not removed by dialysis (see section 5.2). Treatment of overdose should be directed according to local standard medical practice. The expected effect of ticagrelor overdose is an increased risk of bleeding, related to platelet inhibition. It is unlikely that platelet transfusion would be of clinical benefit in patients with bleeding (see section 4.4). If bleeding occurs, other appropriate supportive treatment should be given.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic Properties
Therapeutic class: platelet aggregation inhibitors, excluding heparin, ATC code: B01AC24
Mechanism of action
Ticagrelor Holsten contains ticagrelor, which belongs to the chemical class of cyclopentyltriazolopyrimidines (CPTP).
Ticagrelor is an oral, direct-acting, selective, and reversibly binding P2Y12 receptor antagonist, which prevents ADP-mediated activation and aggregation of platelets associated with the P2Y12 receptor.
Ticagrelor does not prevent ADP binding but, after binding to the P2Y12 receptor, prevents ADP-stimulated signal transduction. Since platelets play a critical role in the initiation and/or progression of atherothrombotic complications, it has been shown that inhibition of platelet activation and aggregation reduces the risk of cardiovascular events, such as death, myocardial infarction, or stroke.
Ticagrelor also increases local concentrations of endogenous adenosine by inhibiting the equilibrative nucleoside transporter 1 (ENT-1).
It has been shown that ticagrelor enhances the following adenosine-mediated effects in healthy volunteers and patients with coronary artery disease: vasodilation (measured as an increase in coronary flow in healthy volunteers and patients with coronary artery disease; headache), inhibition of platelet activation (in human blood in vitro), and dyspnea. However, the relationship between the observed increase in adenosine levels and clinical effects (e.g., morbidity-mortality) has not been clearly established.
Pharmacodynamic effects
Onset of action
In patients with stable coronary artery disease treated with acetylsalicylic acid, ticagrelor exhibits a rapid onset of pharmacological action, as evidenced by a mean inhibition of platelet aggregation (IPA) of approximately 41% after 30 minutes of ticagrelor loading dose of 180 mg, with a maximum IPA effect of 89% after 2 to 4 hours of treatment, which is sustained from 2 to 8 hours. In 90% of patients, the highest level of platelet inhibition, exceeding 70%, is observed after 2 hours of treatment.
Offset of action
If a CABG is planned, the risk of bleeding associated with ticagrelor is greater than with clopidogrel when discontinuing therapy less than 96 hours before surgery.
Switching therapy
Switching from clopidogrel 75 mg to ticagrelor 90 mg twice daily results in an increase in IPA of 26.4% in absolute terms, and switching from ticagrelor to clopidogrel results in a decrease in IPA of 24.5% in absolute terms. Patients can switch from clopidogrel to ticagrelor without interruption of antiplatelet effect (see section 4.2).
Clinical efficacy and safety in clinical trials
Clinical data supporting the efficacy and safety of ticagrelor come from two Phase 3 studies:
- the PLATO study [ PLATelet Inhibition and Patient Outcomes], in which ticagrelor was compared to clopidogrel, with both drugs given in combination with ASA (acetylsalicylic acid) and other standard treatments;
- the PEGASUS TIMI-54 study [ PrEvention with TicaGrelor of SecondAry Thrombotic Events inHigh-RiSk AcUte Coronary Syndrome Patients], in which ticagrelor plus ASA was compared to ASA alone.
PLATO study (acute coronary syndromes)
The PLATO study included 18,624 patients with acute coronary syndrome who presented within 24 hours of symptom onset with unstable angina (UA), non-ST-elevation myocardial infarction (NSTEMI), or ST-elevation myocardial infarction (STEMI) and who were initially treated medically, or had undergone percutaneous coronary intervention (PCI), or had undergone coronary artery bypass grafting (CABG).
Clinical efficacy
In combination with a daily dose of ASA, ticagrelor 90 mg twice daily demonstrated superiority over clopidogrel 75 mg daily in preventing the composite endpoint of cardiovascular death, myocardial infarction, or stroke, with the difference driven primarily by cardiovascular death and myocardial infarction. Patients received clopidogrel loading dose of 300 mg (or 600 mg in patients undergoing PCI) or ticagrelor loading dose of 180 mg.
This result was achieved early (absolute risk reduction [ARR] 0.6% and relative risk reduction [RRR] 12% at 30 days), and the efficacy of treatment was sustained over the 12-month period, achieving an ARR of 1.9% over 12 months and an RRR of 16%. These results indicate that the appropriate treatment duration with ticagrelor 90 mg twice daily is 12 months (see section 4.2).
Treatment of 54 patients with acute coronary syndrome with ticagrelor instead of clopidogrel prevents 1 cardiovascular event; treatment of 91 patients prevents 1 cardiovascular death (see Figure 1 and Table 4).
Better outcomes with ticagrelor compared to clopidogrel were consistently observed across many pre-specified subgroups of patients, including body mass index, sex, diabetes in history, transient ischemic attack, or revascularization; concomitant treatment with heparin, glycoprotein IIb/IIIa inhibitors, or proton pump inhibitors (see section 4.5); final clinical diagnosis (STEMI, NSTEMI, or UA) and planned treatment strategy at the time of randomization (invasive or conservative strategy).
With minimal significance, the treatment effect differed according to region, with the hazard ratio (HR) for the primary endpoint indicating a benefit of ticagrelor across the world, except for North America, which accounted for approximately 10% of the overall study population, where the HR favored clopidogrel (interaction p=0.045).
Factor analyses suggest a possible relationship with the ASA dose, indicating that the efficacy of ticagrelor may be reduced with increasing ASA doses. ASA doses for long-term administration with ticagrelor should be 75-150 mg (see sections 4.2 and 4.4).
Figure 1 shows the estimated risk of first occurrence of any component of the composite endpoint for efficacy evaluation.
Figure 1 - Analysis of the primary clinical composite endpoint of cardiovascular death, myocardial infarction, and stroke (PLATO)
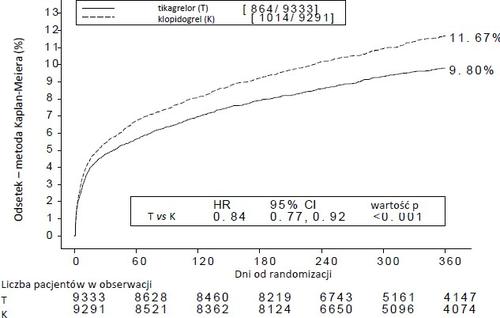
Ticagrelor reduced the incidence of the primary composite endpoint compared to clopidogrel, in both the UA/NSTEMI and STEMI patient groups (Table 4). Therefore, the medicinal product Ticagrelor Holsten 90 mg twice daily in combination with ASA in low doses can be used in patients with acute coronary syndrome (unstable angina, non-ST-elevation myocardial infarction [NSTEMI], or ST-elevation myocardial infarction [STEMI]), including patients treated medically and those undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).
Table 4 - Analysis of primary and secondary endpoints for efficacy evaluation (PLATO)
| Ticagrelor 90 mg twice daily (% of patients with event) N=9333 | Clopidogrel 75 mg daily (% of patients with event) N=9291 | ARRa (%/year) | RRRa (%) (95% CI) | p-value |
| Event occurred) N=9333 | Event occurred) N=9291 | ||||
| Cardiovascular death, MI (excluding silent MI), or stroke | 8.5 | 10.0 | 1.7 | 16 (6, 25) | 0.0025 |
| Planned invasive strategy | 8.5 | 10.0 | 1.7 | 16 (6, 25) | 0.0025 |
| Planned conservative strategy | 11.3 | 13.2 | 2.3 | 15 (0.3, 27) | 0.0444 |
| Cardiovascular death | 3.8 | 4.8 | 1.1 | 21 (9, 31) | 0.0013 |
| MI (excluding silent MI) | 5.4 | 6.4 | 1.1 | 16 (5, 25) | 0.0045 |
| Stroke | 1.3 | 1.1 |
|
| 0.2249 |
| Death from any cause, MI (excluding silent MI), or stroke | 9.7 | 11.5 | 2.1 | 16 (8, 23) | 0.0001 |
| Cardiovascular death, total MI, stroke, SRI, RI, TIA, or other ATE | 13.8 | 15.7 | 2.1 | 12 (5, 19) | 0.0006 |
| Death from any cause | 4.3 | 5.4 | 1.4 | 22 (11, 31) | 0.0003 |
| Stent thrombosis | 1.2 | 1.7 | 0.6 | 32 (8, 49) | 0.0123 |
Genetic subanalysis in the PLATO study
Genotyping for CYP2C19 and ABCB1, performed in the PLATO study in 10,285 patients, allowed for the assessment of the relationship between genotype groups and the results of the PLATO study.
The superiority of ticagrelor over clopidogrel in reducing the incidence of major cardiovascular events was not significantly dependent on the CYP2C19 or ABCB1 genotype. As in the overall PLATO study, the total number of major bleedings according to the PLATO definition did not differ between the ticagrelor and clopidogrel groups, regardless of the CYP2C19 or ABCB1 genotype. Major bleedings according to the PLATO definition, not related to CABG, occurred more frequently in the ticagrelor group compared to the clopidogrel group in patients with loss of one or more functional CYP2C19 alleles, but similarly to the clopidogrel group in patients without loss of functional alleles.
Overall assessment of efficacy and safety
The overall assessment of efficacy and safety (cardiovascular death, myocardial infarction, stroke, or major bleeding according to the PLATO definition) indicates that the benefits of ticagrelor, compared to clopidogrel, are not lost due to the number of major bleedings (ARR 1.4%, RRR 8%, HR 0.92; p=0.0257) over the 12-month period following acute coronary syndrome.
Clinical safety
Subgroup with Holter monitoring
To investigate the occurrence of ventricular pauses and other arrhythmias during the PLATO study, investigators monitored a subgroup of approximately 3,000 patients with Holter monitoring, with about 2,000 recordings obtained in the acute phase of acute coronary syndrome and after 1 month. The primary variable observed was the occurrence of ventricular pauses ≥3 seconds. A higher number of ventricular pauses was observed in the ticagrelor group (6.0%) than in the clopidogrel group (3.5%) in the acute phase of acute coronary syndrome; and after 1 month, 2.2% and 1.6%, respectively (see section 4.4). The increased incidence of ventricular pauses in the acute phase of acute coronary syndrome was more pronounced in patients treated with ticagrelor with a history of heart failure (9.2% versus 5.4% of patients without heart failure in history; in the case of clopidogrel, 4.0% of patients with heart failure in history and 3.6% of patients without heart failure in history). This imbalance did not occur after 1 month: 2% versus 2.1% in patients treated with ticagrelor, with or without heart failure, respectively; and 3.8% versus 1.4% in patients treated with clopidogrel. No adverse clinical consequences were associated with these abnormalities (including the use of a pacemaker) in this patient group.
PEGASUS study (myocardial infarction in history)
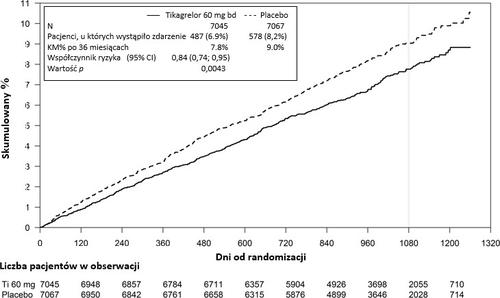
The PEGASUS TIMI-54 study was a randomized, double-blind, parallel-group, placebo-controlled, multinational, multicenter study evaluating the prevention of cardiovascular events with ticagrelor in 2 doses (either 90 mg twice daily or 60 mg twice daily) in combination with ASA in low doses (75-150 mg) compared to ASA alone in patients with a history of myocardial infarction and additional risk factors for cardiovascular events.
Patients were eligible for the study if they were at least 50 years old, had a history of myocardial infarction (1-3 years before randomization), and had at least one of the following risk factors for atherothrombotic events: age ≥65 years, diabetes requiring pharmacological treatment, a second myocardial infarction, multivessel coronary artery disease, or chronic non-end-stage renal disease.
Patients were not eligible for the study if they were planned to receive a P2Y12 receptor antagonist, dipyridamole, cilostazol, or anticoagulant therapy during the study; if they had a history of bleeding disorder or had ischemic stroke or intracranial hemorrhage, brain tumor, or arteriovenous malformation; or if they had gastrointestinal bleeding within the last 6 months or had undergone a major surgical procedure within the last 30 days.
Clinical efficacy
Figure 2 - Analysis of the primary clinical composite endpoint of cardiovascular death, myocardial infarction, and stroke (PEGASUS)
5.2 Pharmacokinetic properties
Ticagrelor exhibits linear pharmacokinetics, and exposure to ticagrelor and its active metabolite (AR-C124910XX) increases approximately in proportion to dose over the dose range of 1260 mg.
Absorption
Ticagrelor is rapidly absorbed, with a median tmax of approximately 1.5 hours. The formation of the main circulating metabolite AR-C124910XX (also active) from ticagrelor is rapid, with a median tmax of approximately 2.5 hours. After administration of a single 90 mg oral dose of ticagrelor to healthy volunteers in the fasted state, Cmax is 529 ng/ml and AUC is 3451 ng*h/ml. For the metabolite related to the parent compound, the ratios are 0.28 for Cmax and 0.42 for AUC.
The pharmacokinetics of ticagrelor and AR-C124910XX in patients with a history of myocardial infarction were essentially similar to those observed in the ACS population. According to a population pharmacokinetic analysis in the PEGASUS study, the median ticagrelor Cmax was 391 ng/ml and AUC was 3801 ng*h/ml at steady state after administration of ticagrelor 60 mg twice daily. For ticagrelor 90 mg twice daily, Cmax was 627 ng/ml and AUC was 6255 ng*h/ml at steady state.
The mean absolute bioavailability of ticagrelor is estimated to be 36%. Consumption of a high-fat meal results in a 21% increase in ticagrelor AUC and a 22% decrease in Cmax of the active metabolite, but does not significantly alter ticagrelor Cmax and AUC of the active metabolite. It is considered that these small changes are of minimal clinical significance, and therefore ticagrelor can be administered with or without food. Both ticagrelor and the active metabolite are substrates for the glycoprotein P (P-gp).
Ticagrelor, when crushed and mixed with water, administered orally or via a nasogastric tube, has bioavailability comparable to that of the whole tablet, in terms of AUC and Cmax for ticagrelor and the active metabolite. The initial exposure (0.5 and 1 hour after administration) of ticagrelor, when administered as crushed tablets mixed with water, was higher than with the whole (uncrushed) tablet, with essentially identical concentration profiles at later times (from 2 to 48 hours).
Distribution
The volume of distribution at steady state is 87.5 l. Ticagrelor and the active metabolite are extensively bound to human plasma proteins (>99.0%).
Metabolism
CYP3A4 is the primary enzyme responsible for the metabolism of ticagrelor and the formation of its active metabolite, and interactions with other substrates of the CYP3A4 isoenzyme include both activation and inhibition.
The main metabolite of ticagrelor, AR-C124910XX, is also active, as demonstrated in vitro studies where it binds to the platelet ADP receptor P2Y.
Systemic exposure to the active metabolite is approximately 30-40% of the exposure to ticagrelor.
Elimination
The primary route of elimination of ticagrelor is hepatic metabolism. After administration of radiolabeled ticagrelor, the mean recovery of radioactivity was approximately 84% (57.8% in feces and 26.5% in urine). Recovered ticagrelor and its active metabolite in urine accounted for less than 1% of the dose in both cases. The main route of elimination of the active metabolite is likely to be biliary excretion. The mean half-life is approximately 7 hours for ticagrelor and 8.5 hours for the active metabolite.
Special populations
Elderly
In population pharmacokinetic analyses, elderly patients (≥75 years) with ACS showed higher exposure to ticagrelor (approximately 25% for Cmax and AUC) and the active metabolite compared to younger patients. These differences are not considered to be clinically significant (see section 4.2).
Children and adolescents
There are limited data available in children and adolescents with sickle cell disease (see sections 4.2 and 5.1).
In the HESTIA 3 study, patients aged 2 to less than 18 years, weighing ≥12 to ≤24 kg, >24 to ≤48 kg, and >48 kg, received ticagrelor as 15 mg oral dispersible tablets at doses of 15, 30, and 45 mg twice daily, respectively. A population pharmacokinetic analysis showed that mean AUC ranged from 1095 ng*h/ml to 1458 ng*h/ml, and mean Cmax ranged from 143 ng/ml to 206 ng/ml at steady state.
Sex
Women showed higher exposure to ticagrelor and the active metabolite than men. These differences are not considered to be clinically significant.
Renal impairment
In patients with severe renal impairment (creatinine clearance <30 ml min), exposure to ticagrelor was approximately 20% lower, while the active metabolite 17% higher than in patients with normal renal function.
In patients with end-stage renal disease on hemodialysis, ticagrelor 90 mg administered on a non-dialysis day showed AUC and Cmax values 38% and 51% higher, respectively, than in patients with normal renal function. Similar increases in exposure were observed when ticagrelor was administered immediately prior to dialysis (38% and 61%, respectively), indicating that ticagrelor is not dialyzable. Exposure to the active metabolite increased to a lesser extent (AUC by 13-14% and Cmax by 17-36%). The effect of ticagrelor on platelet aggregation was independent of dialysis in patients with end-stage renal disease and similar to that in patients with normal renal function (see section 4.2).
Hepatic impairment
Cmax and AUC of ticagrelor were 12% and 23% higher, respectively, in patients with mild hepatic impairment compared to matched healthy subjects; however, the effect of ticagrelor on platelet aggregation was similar in both groups. No dose adjustment is needed in patients with moderate hepatic impairment. Studies have not been conducted in patients with severe hepatic impairment, and no information is available on the pharmacokinetics of ticagrelor in this population. In patients with baseline moderate or severe elevations in one or two liver function tests, plasma concentrations of ticagrelor were similar or slightly higher than in patients without baseline elevations in these tests. No dose adjustment is needed in patients with moderate hepatic impairment (see sections 4.2 and 4.4).
Racial differences
Asian patients showed approximately 39% higher mean bioavailability than Caucasian patients. In black patients, bioavailability of ticagrelor was 18% lower than in Caucasian patients. In clinical pharmacology studies in Japanese subjects, approximately 40% higher (and 20% higher after adjustment for body weight) exposure to ticagrelor (Cmax and AUC) was observed compared to Caucasian subjects. Exposure in Hispanic patients was similar to that in Caucasian patients.
5.3 Preclinical safety data
Preclinical data from conventional studies of safety pharmacology, single and repeated dose toxicity, and genotoxicity of ticagrelor and its main metabolite did not indicate a safety risk for humans.
After exposure under clinical conditions to several animal species, gastrointestinal irritation was observed (see section 4.8).
In female rats given high doses of ticagrelor, an increased incidence of uterine tumors (adenocarcinomas) and an increased incidence of liver adenomas were observed. The mechanism of uterine tumor formation in rats is likely to be related to hormonal imbalance, which may lead to tumor formation in rats. The mechanism of liver adenoma formation is likely to be related to increased enzymatic activity in the liver, specific to rodents. Therefore, it is considered unlikely that these tumors are relevant to humans.
Minor developmental abnormalities were observed in rats given toxic doses to pregnant females (safety margin 5.1). In rabbit fetuses, minor delays in liver maturation and skeletal development were observed when pregnant females were given high doses without signs of toxicity in the pregnant females (safety margin 4.5).
Studies in rats and rabbits showed toxic effects on reproduction, with minor reductions in maternal body weight gain, decreased fetal survival, lower birth weight, and delayed growth. Ticagrelor caused irregular cycles (mostly prolonged) in female rats, but did not affect overall fertility in male and female rats. Pharmacokinetic studies conducted with radiolabeled ticagrelor showed that both the parent compound and its metabolites cross the placenta into the milk of rats (see section 4.6).
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Tablet core
Hypromellose (E464)
Mannitol (E421)
Microcrystalline cellulose (E460)
Sodium carboxymethyl cellulose
Magnesium stearate (E470b)
Tablet coating
Hypromellose (E464)
Titanium dioxide (E171)
Macrogol 400 (E1521)
Talc (E553b)
Iron oxide red (E172)
6.2 Incompatibilities
None.
6.3 Shelf life
3 years
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions; store in the original package to protect from light.
6.5 Nature and contents of container
Transparent PVC/PVDC/Aluminum and/or transparent PVC/PE/PVDC/Aluminum blisters in a cardboard box.
Blisters (with sun/moon symbols or without) in cardboard boxes of 14, 15, 20, 28, 30, 56, 60, 90, 98, 100, 168, 195, 196, and 200 film-coated tablets.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
Holsten Pharma GmbH
Hahnstraße 31-35
60528 Frankfurt am Main
Germany
8. MARKETING AUTHORISATION NUMBERS
Authorisation number:
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
DATE OF REVISION OF THE TEXT
Date of first authorisation:
10. DATE OF REVISION OF THE TEXT
CHARACTERISTICS OF THE MEDICINAL PRODUCT
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGenepharm S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ticagrelor HolstenDosage form: Tablets, 60 mgActive substance: ticagrelorPrescription requiredDosage form: Tablets, 90 mgActive substance: ticagrelorPrescription requiredDosage form: Tablets, 60 mgActive substance: ticagrelorManufacturer: Krka, d.d., Novo mestoPrescription required
Alternatives to Ticagrelor Holsten in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ticagrelor Holsten in Hiszpania
Alternative to Ticagrelor Holsten in Ukraina
Online doctors for Ticagrelor Holsten
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ticagrelor Holsten – subject to medical assessment and local rules.














