
Thorens
Ask a doctor about a prescription for Thorens

How to use Thorens
Leaflet attached to the packaging: information for the user
THORENS, 10,000 IU/ml, oral drops, solution
Cholecalciferol (Vitamin D)
Read the leaflet carefully before taking the medicine, as it
contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is THORENS and what is it used for
- 2. Important information before taking THORENS
- 3. How to take THORENS
- 4. Possible side effects
- 5. How to store THORENS
- 6. Contents of the pack and other information
1.
What is THORENS and what is it used for
THORENS oral drops, solution contains cholecalciferol (Vitamin D). Vitamin D
is found in some foods and is also produced by the body when the skin is exposed to
sunlight. Vitamin D helps the body absorb calcium in the kidneys and intestines and
supports bone formation. A deficiency of Vitamin D is the main cause of rickets (disrupted
mineralization of bones in children) and osteomalacia (insufficient mineralization of bones
in adults).
THORENS oral drops, solution is used to prevent Vitamin D deficiency and to treat it in
adults, adolescents, and children at risk of Vitamin D deficiency.
THORENS oral drops, solution may also be used as an adjunct in patients taking medicines
for osteoporosis.
2. Important information before taking THORENS
When not to take THORENS:
- if you are allergic to Vitamin D or any of the other ingredients of this medicine (listed in section 6);
- if you have high levels of calcium in your blood (hypercalcemia) or urine (hypercalciuria);
- if you have kidney stones (nephrolithiasis) or severe kidney problems;
- if you have high levels of Vitamin D in your blood (hypervitaminosis D).
Warnings and precautions
Before taking THORENS, discuss it with your doctor or pharmacist if:
- you are taking certain medicines for heart conditions (e.g., digitalis glycosides, such as digoxin);
- you have sarcoidosis (an immune system disease that can cause high levels of Vitamin D in the body);
- you are taking medicines containing Vitamin D or foods rich in Vitamin D, such as some types of fortified milk;
- you are exposed to large amounts of sunlight during THORENS treatment;
- you are taking additional calcium supplements. Your doctor will monitor your blood calcium levels to ensure they are not too high during THORENS treatment;
- you have kidney damage or disease. Your doctor may decide to monitor your blood or urine calcium levels;
- you have been taking Vitamin D for a long time at a daily dose exceeding 1000 IU (1000 international units). Your doctor should monitor your blood calcium levels through laboratory tests.
THORENS with food, drink, and alcohol
It is best to take this medicine with a large meal to support the absorption of Vitamin D by the body. To facilitate the intake of this medicine, the drops can be mixed with cold or warm food. For more information, see section 3 "How to take THORENS".
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before taking this medicine. THORENS can be used during pregnancy and breastfeeding only if prescribed by a doctor.
In pregnancy, do not exceed the recommended dose, as prolonged hypercalcemia can lead to physical and mental developmental delays in the child, as well as supravalvular aortic stenosis and retinopathy.
Driving and using machines
Information on the possible effect of this medicine on the ability to drive and use machines is limited. However, it is not expected to affect the ability to drive and use machines.
3. How to take THORENS
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Before use, shake the bottle.
THORENS is best taken with a large meal.
This medicine has the taste of olive oil. It can be taken as is or, as prescribed by your doctor, the prescribed number of drops can be mixed with a spoon or a small amount of cold or warm food just before administration. Ensure that the entire dose is taken.
Administration in adults
The recommended dose for the prevention of Vitamin D deficiency and as an adjunct in patients taking medicines for osteoporosis is 3 to 4 drops (600-800 IU) per day.
In the treatment of Vitamin D deficiency, the usual dose is 4 drops (800 IU) per day.
Higher doses must be adjusted according to the desired level of 25-hydroxycholecalciferol (25(OH)D) in serum, disease severity, and patient response to treatment.
The daily dose should not exceed 4000 IU (20 drops per day).
Administration in children and adolescents
In the prevention of Vitamin D deficiency in children (from 0 to 11 years old) with a confirmed risk factor, the recommended dose is 2 DROPS(400 IU) per day. In the prevention of Vitamin D deficiency in adolescents (from 12 to 18 years old) with a confirmed risk factor, the recommended dose is from 3 to 4 drops (600-800 IU) per day.
In the treatment of Vitamin D deficiency in children (from 0 to 11 years old) and adolescents (from 12 to 18 years old), the dose should be adjusted according to the desired level of 25-hydroxycholecalciferol (25(OH)D) in serum, disease severity, and patient response to treatment. The daily dose should not exceed 1000 IU in infants under 1 year, 2000 IU in children from 1 to 10 years, and 4000 IU in adolescents.
In children, THORENS can be mixed with a small amount of baby food, yogurt, milk, cheese, or other dairy products. The medicine should not be mixed with bottle-fed milk or soft foods in containers, as if the child does not consume the entire portion, they will not receive the full dose of the medicine. Ensure that the entire dose is taken. In children who are no longer breastfed, the prescribed dose of the medicine should be given during a fairly large meal.
Administration during pregnancy and breastfeeding
The recommended dose is 400 IU/day (2 drops). Higher doses may be required after confirmation of Vitamin D deficiency, but do not exceed the recommended dose prescribed by your doctor.
Instructions for using the bottle with a dropper
The packaging contains 1 bottle and a dropper with a cap. The bottle is closed with a plastic cap with a child-resistant closure. The dropper is protected by a plastic cover. Before use, shake the bottle and follow the instructions below:
a.
To open the bottle, press and turn the plastic cap (see Figure 1).
b.
Remove the plastic cover protecting the glass body of the dropper (see Figure 2).
c.
Insert the glass tip of the dropper into the bottle to draw the contents. Release the prescribed number of drops onto a spoon.
d.
To close the bottle, remove the dropper and then screw the plastic cap (see Figure 3).
e.
Carefully screw the plastic cover onto the dropper to protect the glass body.
f.
Place the closed bottle and the dropper with the plastic cover back in the original cardboard box.
Figure 1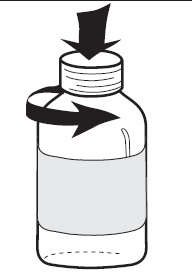 | Figure 2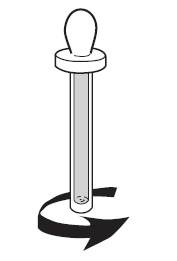 | Figure 3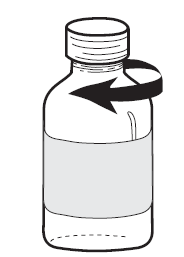 |
Instructions for using the container with a dropper
The box contains a container with a dropper. The container with a dropper is closed with a child-resistant plastic cap. The instructions for opening and using are below:
a.
To open the container with a dropper, press and turn the plastic cap.
b.
Turn the container with a dropper upside down and release the prescribed number of drops onto a spoon.
c.
After administering the drops, turn the container with a dropper back to its original position.
d.
To close the container with a dropper, screw the plastic cap.
e.
Place the container with a dropper back in the original cardboard box.
Taking a higher dose of THORENS than recommended
If you take more THORENS than prescribed, stop taking it and contact your doctor. If you cannot talk to your doctor, go to the emergency department of the nearest hospital, taking this leaflet with you.
The most common symptoms of overdose are: nausea, vomiting, increased thirst, production of a large amount of urine per day, constipation, and dehydration, high levels of calcium in the blood and urine (hypercalcemia and hypercalciuria) found in laboratory tests.
Missing a dose of THORENS
If you miss a dose of THORENS, take it as soon as possible. Take the next dose at the usual time. However, if it is almost time for the next dose, do not take the missed dose, just take the next dose at the usual time.
Do not take a double dose to make up for a missed dose.
If you have any further questions about taking this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, THORENS can cause side effects, although not everybody gets them.
Possible side effects reported during THORENS treatment may include:
Uncommon(in less than 1 in 100 people)
- high levels of calcium in the blood (hypercalcemia)
- high levels of calcium in the urine (hypercalciuria)
Rare(in less than 1 in 1000 people)
- skin rash
- itching
- hives
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. You can also report side effects directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw
phone: +48 22 49 21 301
fax: +48 22 49 21 309
email: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store THORENS
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and container after EXP. The expiry date refers to the last day of that month.
Do not store above 30°C.
Do not refrigerate or freeze.
Keep the container in the outer packaging to protect from light.
After first opening the container: the product can be stored for a maximum of 6 months.
Do not use this medicine if you notice the solution has become cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What THORENS contains
The active substance is cholecalciferol (Vitamin D).
1 ml of oral solution (50 drops) contains 10,000 IU of cholecalciferol (Vitamin D), which corresponds to 0.25 mg.
1 drop contains 200 IU of cholecalciferol (Vitamin D).
The other ingredient is purified olive oil.
What THORENS looks like and contents of the pack
THORENS, 10,000 IU, oral drops, solution is a clear and colorless to greenish-yellow oily solution without visible particles and/or sediment. It may be supplied in the following pack sizes:
Bottle with a dropper
A 20 ml brown glass bottle closed with a child-resistant plastic cap.
Each pack contains 1 brown glass bottle with 10 ml of solution (corresponding to 500 drops) and 1 dropper with a cover (marked CE 0068).
Container with a dropper
A 20 ml brown glass container with a dropper closed with a child-resistant plastic cap.
Each pack contains 1 brown glass container with a dropper with 10 ml of solution (corresponding to 500 drops).
Marketing authorization holder
Italfarmaco S.p.A.
Viale Fulvio Testi, 330
20126 Milan
Italy
Manufacturer
Abiogen Pharma S.p.A.
Via Meucci, 36 Loc. Ospedaletto
56121 Pisa
Italy
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Sweden
Deltius 10,000 IE/ml oral drops, solution
Norway
Deltius 10,000 I.E. /ml mixture, solution
Belgium
Thorens 10,000 UI/ml, oral solution in drops
United Kingdom
Thorens 10,000 I.U/ml, oral drops, solution
Germany
Thorens 10,000 IE /ml drops for oral use, solution
Ireland
Thorens 10,000 I.U/ml, oral drops, solution
Netherlands
Thorens 10,000 IE/ml drops for oral use, solution
Spain
Thorens
Portugal
Thorens
Date of last revision of the leaflet:2022-01-13
Other sources of information
Detailed information about this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAbiogen Pharma S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ThorensDosage form: Tablets, 1000 IUActive substance: colecalciferolPrescription requiredDosage form: Tablets, 7000 IUActive substance: colecalciferolPrescription requiredDosage form: Tablets, 30,000 IUActive substance: colecalciferolPrescription required
Alternatives to Thorens in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Thorens in Spain
Alternative to Thorens in Ukraine
Online doctors for Thorens
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Thorens – subject to medical assessment and local rules.














