
Tantum Verde Forte

Ask a doctor about a prescription for Tantum Verde Forte

How to use Tantum Verde Forte
Leaflet attached to the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Tantum Verde Forte
3 mg/ml, spray for oral and throat use
Benzydamine hydrochloride
It is necessary to carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
In case of doubt, consult a doctor or pharmacist.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If there is no improvement after 7 days or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet:
- 1. What is Tantum Verde Forte and what is it used for
- 2. Important information before using Tantum Verde Forte
- 3. How to use Tantum Verde Forte
- 4. Possible side effects
- 5. How to store Tantum Verde Forte
- 6. Package contents and other information
1. What is Tantum Verde Forte and what is it used for
Tantum Verde Forte contains the active substance benzydamine, which belongs to the group of non-steroidal anti-inflammatory drugs (NSAIDs).
Benzydamine has strong anti-inflammatory and analgesic effects and acts locally as an anesthetic and antiseptic. Due to its good local absorption and high concentration in inflamed tissues, symptoms quickly subside. The medicine is well-tolerated and suitable for local treatment of inflammatory symptoms.
The medicine is used to treat symptoms (pain, redness, swelling) associated with inflammation of the oral cavity and throat, such as bacterial and viral infections, laryngitis, inflammation of the mucous membranes after radiotherapy, post-operative conditions in laryngology and stomatology, and after intubation.
2. Important information before using Tantum Verde Forte
When not to use Tantum Verde Forte
If the patient is allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Tantum Verde Forte, consult a doctor or pharmacist.
Page 1 5
Using, especially long-term, locally acting medicines may cause an allergic reaction. In such a case, the medicine should be discontinued and a doctor consulted, who will recommend appropriate action.
Tantum Verde Forte and other medicines
Inform the doctor or pharmacist about all medicines currently or recently used by the patient, as well as any planned to be used.
No interactions of Tantum Verde Forte with other medicines are known.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, suspects she may be pregnant, or plans to have a child, she should consult a doctor or pharmacist before using this medicine.
The doctor will decide on the possibility of using the medicine in pregnant and breastfeeding women.
Driving and operating machinery
The medicine does not affect the ability to drive or operate machinery.
Tantum Verde Forte contains methyl parahydroxybenzoate (E 218), hydrogenated castor oil.
The medicine may cause allergic reactions (possible late reactions).
The medicine may cause skin reactions.
3. How to use Tantum Verde Forte
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist. In case of doubt, consult a doctor or pharmacist.
The medicine is intended for use in adults, especially those who cannot rinse their mouths.
The medicine should be used 2 to 6 times a day, 2-4 doses.
Each dose contains 0.17 ml of solution.
Do not use a larger dose than recommended.
The medicine is intended for short-term use. Continuous treatment should not exceed 7 days. If symptoms recur or worsen, consult a doctor.
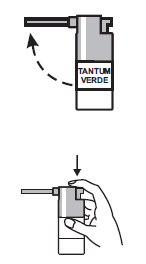
Instructions for use:
- 1. Lift the nozzle.
- 2. Place the nozzle in the mouth and direct its outlet towards the area of inflammation in the oral cavity or throat.
- 3. Press the ridged surface of the pump firmly with your finger until the dose of medicine is released. Repeat this action the appropriate number of times.
Note: When using the medicine for the first time, press the button several times to fill the pump and obtain proper spraying.
If you feel that the effect of the medicine is too strong or too weak, consult a doctor.
Using a larger dose of Tantum Verde Forte than recommended
Page 2 5
In case of using a larger dose than recommended or accidental ingestion of a large amount of medicine, immediately contact a doctor or pharmacist. Bring this leaflet with you.
Missing a dose of Tantum Verde Forte
Do not use a double dose to make up for a missed dose.
In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Uncommon
(less than 1 in 100 people, but more than 1 in 1000 people):
skin damage due to hypersensitivity to light (phototoxic reactions).
Rare
(less than 1 in 1000 people, but more than 1 in 10,000 people):
burning or dryness in the mouth.
Very rare
(less than 1 in 10,000 people):
laryngospasm, angioedema.
Frequency not known
(cannot be estimated from the available data):
- allergic reaction (hypersensitivity),
- severe allergic reaction (anaphylactic shock), which may include difficulty breathing, chest pain, and (or) dizziness, severe itching of the skin or hives, swelling of the face, lips, tongue, and (or) throat, and which may potentially be life-threatening.
- nausea, vomiting, numbness of the mouth, dizziness, headaches.
Local symptoms are related to the pharmacodynamic effect of benzydamine, which, among other things, has a local anesthetic effect.
Local side effects are usually transient, resolve on their own, and rarely require additional treatment.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Tantum Verde Forte
Page 3 5
The medicine should be stored out of sight and reach of children.
There are no special storage recommendations.
After opening, use within 6 months.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and other information
What Tantum Verde Forte contains
- The active substance of the medicine is benzydamine hydrochloride. 100 ml of solution contains 0.3 g of benzydamine hydrochloride.
- The other ingredients are: glycerol, ethanol 96%, sodium saccharin, methyl parahydroxybenzoate (E 218), peppermint flavor, hydrogenated castor oil, purified water.
What Tantum Verde Forte looks like and what the package contains
Tantum Verde Forte is a solution with a peppermint flavor, intended for spraying in the oral cavity and throat.
The package is an HDPE bottle containing 15 ml of solution, equipped with a dosing pump.
The bottle is in a cardboard box.
For more detailed information, contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Lithuania, country of export:
Angelini Pharma Österreich GmbH
Brigittenauer Lände 50-54
1200 Vienna
Austria
Manufacturer
A.C.R.A.F. S.p.A
Via Vecchia del Pinocchio 22
60131 Ancona
Italy
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Lithuanian, country of export, authorization number: LT/1/01/0257/006
Parallel import authorization number: 77/18
Page 4 5
Date of leaflet approval: 07.02.2023
[information about trademark]
Page 5 5
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Angelini Pharma Österreich GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tantum Verde ForteDosage form: Aerosol, 1.5 mg/mlActive substance: benzydaminePrescription not requiredDosage form: Aerosol, 3 mg/mlActive substance: benzydamineManufacturer: Laboratorios Bohm SA Laboratorium Sanitatis S.L. MCM Klosterfrau Vertriebsgesellschaft mbHPrescription not requiredDosage form: Lozenge, 3 mgActive substance: benzydaminePrescription not required
Alternatives to Tantum Verde Forte in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tantum Verde Forte in Spain
Alternative to Tantum Verde Forte in Ukraine
Online doctors for Tantum Verde Forte
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tantum Verde Forte – subject to medical assessment and local rules.






