

Tantum Verde

Ask a doctor about a prescription for Tantum Verde

How to use Tantum Verde
LEAFLET INCLUDED IN THE PACKAGING: INFORMATION FOR THE PATIENT
Warning! Keep the leaflet, the information on the immediate packaging is in a foreign language!
Tantum Verde
1.5 mg/ml, spray for use in the mouth and throat
Benzydamine hydrochloride
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor or pharmacist.
- You should keep this leaflet so that you can read it again if you need to.
- If you need advice or additional information, you should consult a pharmacist.
- If you experience any side effects, including any possible side effects not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
- If after 7 days there is no improvement or the patient feels worse, you should contact a doctor.
Table of contents of the leaflet:
- 1. What is Tantum Verde and what is it used for
- 2. Important information before using Tantum Verde
- 3. How to use Tantum Verde
- 4. Possible side effects
- 5. How to store Tantum Verde
- 6. Contents of the packaging and other information
1. What is Tantum Verde and what is it used for
Tantum Verde, a spray for use in the mouth and throat, contains the active substance benzydamine, which belongs to the group of indole non-steroidal anti-inflammatory drugs (NSAIDs) for local and general use.
Benzydamine is characterized by its anti-inflammatory and analgesic effects, and it also has a local anesthetic and disinfectant effect. The medicine is well absorbed locally, reaching high concentrations in the tissues affected by the inflammatory process. The medicine is generally well tolerated and is suitable for local treatment of inflammation symptoms.
The medicine is for use on the mucous membrane of the mouth and throat.
The medicine is used to treat symptoms (pain, redness, swelling) associated with inflammation of the mouth and throat, such as bacterial and viral infections, inflammation of the mucous membranes after radiotherapy, post-operative conditions in laryngology and stomatology, as well as after intubation.
If after 7 days there is no improvement or the patient feels worse, you should consult a doctor.
2. Important information before using Tantum Verde
When not to use Tantum Verde
- if the patient is allergic to benzydamine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Tantum Verde, you should discuss it with your doctor or pharmacist.
Using, especially long-term, medicines with local action may cause an allergic reaction. In such a case, the medicine should be discontinued and a doctor should be consulted, who will recommend appropriate action.
Tantum Verde and other medicines
You should tell your doctor or pharmacist about all medicines that the patient is currently using or has recently used, as well as about medicines that the patient plans to use.
No interactions of Tantum Verde with other medicines are known.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, suspects that she may be pregnant, or plans to have a child, she should consult a doctor or pharmacist before using this medicine.
Driving and using machines
The medicine does not affect the ability to drive or use machines.
Tantum Verde contains methyl parahydroxybenzoate (E 218), ethanol, peppermint flavor, and sodium.
Methyl parahydroxybenzoate (E 218)
The medicine may cause allergic reactions (late-type reactions are possible).
Ethanol
This medicine contains 13.6 mg of alcohol (ethanol) in a single dose of 0.17 ml of solution, which is equivalent to 80 mg/ml. The amount of alcohol in a single dose of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine will not have noticeable effects.
Peppermint flavor
The medicine contains a peppermint flavor with benzyl alcohol (E 1519), cinnamaldehyde, citral, citronellol, geraniol, isoeugenol, linalool, eugenol, and d-limonene, which may cause allergic reactions.
The medicine contains 0.024 mg of benzyl alcohol in each dose (0.17 ml). Benzyl alcohol may cause allergic reactions and mild local irritation.
Administering benzyl alcohol to small children is associated with a risk of serious side effects, including respiratory disorders (so-called "gasping syndrome"). Do not administer to newborns (up to 4 weeks of age) without a doctor's recommendation.
Do not administer to small children (under 3 years of age) for more than a week without a doctor's or pharmacist's recommendation.
Pregnant or breastfeeding women should consult a doctor before using the medicine, as a large amount of benzyl alcohol may accumulate in their body and cause side effects (so-called metabolic acidosis).
Patients with liver or kidney disease should consult a doctor before using the medicine, as a large amount of benzyl alcohol may accumulate in their body and cause side effects (so-called metabolic acidosis).
Sodium
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means that the medicine is considered "sodium-free".
3. How to use Tantum Verde
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor or pharmacist. In case of doubt, you should consult a doctor or pharmacist.
Tantum Verde should be sprayed onto the mucous membrane of the mouth and throat.
The recommended dose is:
Children and adolescents
Children under 6 years of age: 1 dose of the medicine (spray) per 4 kg of body weight, 2 to 6 times a day.
Regardless of body weight, do not exceed 4 doses at a time.
Children between 6 and 12 years of age: 4 doses of the medicine, 2 to 6 times a day.
Children over 12 years of age: 4 to 8 doses of the medicine, 2 to 6 times a day.
Adults
4 to 8 doses (sprays) of the medicine, 2 to 6 times a day.
Each dose of the medicine contains 0.17 ml of solution.
Do not use a higher dose than recommended. Continuous treatment should not be longer than 7 days, and any extension of treatment should be decided by a doctor.
Instructions for use:
- 1. Lift the nozzle.
- 2. Place the nozzle in the mouth and direct its outlet towards the area of inflammation in the mouth or throat.
- 3. Press the ridged surface of the pump firmly with your finger until the dose of the medicine is released. Repeat this action the appropriate number of times.
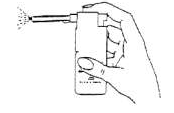
Note: When using the medicine for the first time, the pump button should be pressed several times to fill the pump and obtain the correct spray.
If you feel that the effect of the medicine is too strong or too weak, you should consult a doctor.
Using a higher dose of Tantum Verde than recommended
Overdose of the medicine is unlikely due to the low concentration of the active substance in the medicine and the local route of administration.
No cases of overdose have been observed so far.
In case of using a higher dose than recommended or accidental ingestion of a large amount of the medicine, you should immediately consult a doctor or pharmacist.
Missing a dose of Tantum Verde
You should not use a double dose to make up for a missed dose.
In case of any further doubts related to the use of this medicine, you should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very rare(less than 1 in 10,000 people) and frequency not known(cannot be estimated from the available data):
- allergic reaction (hypersensitivity),
- severe allergic reaction (anaphylactic shock), which may include symptoms such as difficulty breathing, chest pain or tightness, and (or) dizziness or fainting, severe itching of the skin or hives, swelling of the face, lips, tongue, and (or) throat, and which may potentially be life-threatening,
- burning sensation of the mucous membrane of the mouth, dryness of the mouth, nausea, and vomiting, disorders of sensation, numbness, dizziness, and headaches, rash.
Local symptoms are related to the pharmacodynamic effect of benzydamine, which, among other things, has a local anesthetic effect.
Local side effects are usually transient, resolve on their own, and rarely require additional treatment.
Benzydamine used locally is absorbed into the circulation in small amounts, and therefore general side effects occur very rarely.
Reporting side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help provide more information on the safety of the medicine.
5. How to store Tantum Verde
The medicine should be stored out of sight and reach of children.
There are no special precautions for storage.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
Shelf life after first opening – 6 months.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Tantum Verde contains
- The active substance of the medicine is benzydamine hydrochloride. 100 ml of solution contains 0.15 g of benzydamine hydrochloride.
- The other ingredients are: glycerol, ethanol 96%, methyl parahydroxybenzoate (E 218), peppermint flavor (contains, among others, benzyl alcohol (E 1519), cinnamaldehyde, citral, citronellol, geraniol, isoeugenol, linalool, eugenol, d-limonene), saccharin, sodium bicarbonate, polysorbate 20, purified water.
What Tantum Verde looks like and what the packaging contains
Clear, colorless solution with a peppermint flavor.
HDPE container with a dosing pump with a foldable dosing nozzle (nozzle), in a cardboard box.
1 container - 30 ml
In order to obtain more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Lithuania, the country of export:
Angelini Pharma Österreich GmbH
Brigittenauer Lände 50-54
1200 Vienna
Austria
Manufacturer:
A.C.R.A.F. S.p.A
Via Vecchia del Pinocchio 22
60131 Ancona
Italy
Parallel importer:
Medezin Sp. z o.o.
ul. Zbąszyńska 3
91-342 Łódź
Repackaged by:
Medezin Sp. z o.o.
ul. Zbąszyńska 3
91-342 Łódź
Marketing authorization number in Lithuania, the country of export: LT/1/01/0257/005
Parallel import authorization number: 442/19
Date of leaflet approval: 03.12.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Angelini Pharma Österreich GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tantum VerdeDosage form: Aerosol, 1.5 mg/mlActive substance: benzydaminePrescription not requiredDosage form: Aerosol, 3 mg/mlActive substance: benzydamineManufacturer: Laboratorios Bohm SA Laboratorium Sanitatis S.L. MCM Klosterfrau Vertriebsgesellschaft mbHPrescription not requiredDosage form: Lozenge, 3 mgActive substance: benzydaminePrescription not required
Alternatives to Tantum Verde in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tantum Verde in Іспанія
Alternative to Tantum Verde in Україна
Online doctors for Tantum Verde
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tantum Verde – subject to medical assessment and local rules.






