
Starazolin redfree

Ask a doctor about a prescription for Starazolin redfree

How to use Starazolin redfree
Leaflet attached to the packaging: patient information
Starazolin redFREE, 0.5 mg/ml, eye drops, solution
Tetryzoline hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
- If after 2 days there is no improvement or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Starazolin redFREE and what is it used for
- 2. Important information before using Starazolin redFREE
- 3. How to use Starazolin redFREE
- 4. Possible side effects
- 5. How to store Starazolin redFREE
- 6. Contents of the pack and other information
1. What is Starazolin redFREE and what is it used for
Starazolin redFREE is a sterile eye drop solution, preservative-free.
Starazolin redFREE contains tetryzoline hydrochloride as the active substance, which belongs to the group of eye vasoconstrictor drugs.
Starazolin redFREE is indicated for the symptomatic treatment of conditions accompanied by swelling and conjunctival hyperemia, resulting from eye irritation, e.g., by smoke, dust, wind, chlorinated water, cosmetics, as well as in allergic inflammatory conditions, such as hay fever or pollen allergy.
The eye drops alleviate accompanying symptoms, such as burning, itching, pain, excessive tearing, and irritation.
The effect of the medicine starts within a few minutes after administration and lasts for 4 to 8 hours.
2. Important information before using Starazolin redFREE
When not to use Starazolin redFREE
- if the patient is allergic to tetryzoline hydrochloride or any of the other ingredients of this medicine (listed in section 6);
- if the patient has contact hypersensitivity to silver.
- if the patient has narrow-angle glaucoma;
- if the patient has severe heart or blood vessel disease (e.g., coronary heart disease or arterial hypertension);
- if the patient has a pheochromocytoma;
- if the patient has metabolic disorders, e.g., hyperthyroidism, porphyria, or diabetes;
- if the patient is taking monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, or other medicines that may increase blood pressure (see "Starazolin redFREE and other medicines");
- if the patient has benign prostatic hyperplasia (prostate enlargement);
- in children under 2 years of age.
Warnings and precautions
Before starting treatment with Starazolin redFREE, the patient should discuss it with their doctor or pharmacist:
- if the patient has dry conjunctivitis, keratitis, or conjunctivitis;
- if the patient has increased intraocular pressure (glaucoma).
In case of severe eye pain, headache, vision loss, appearance of "floating" spots in the visual field, severe, sharp, or one-sided eye redness, pain when exposed to light, or double vision, the patient should immediately consult a doctor.
Starazolin redFREE should only be used in case of mild eye irritation.
If after 48 hours there is no improvement or the irritation and redness of the eyes persist or worsen, the patient should immediately discontinue the drops and consult a doctor.
Patient using Starazolin redFREE should be aware that eye irritation or redness is often a symptom of a serious eye condition and should consult an ophthalmologist in this regard.
If the eye irritation or redness is due to a severe eye disease, e.g., infection, presence of a foreign body, or chemical damage to the cornea, the patient should immediately consult a doctor.
Prolonged and improper use of this medicine, especially in higher doses than recommended, may reduce its effectiveness and lead to conjunctival redness and persistent nasal congestion.
The patient should be careful not to use a higher dose of this medicine than recommended and not to use it for too long, as prolonged use or abuse may reduce its effectiveness, cause worsening or recurrence of congestion, and lead to conjunctival redness and persistent nasal congestion. The patient should avoid prolonged use, especially in children.
See also section 3 "How to use Starazolin redFREE".
Children
Starazolin redFREE is contraindicated in children under 2 years of age.
The patient should always consult a doctor before using this medicine in children.
Starazolin redFREE and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently using or have recently used, as well as any medicines they plan to use, including those available without a prescription.
Concomitant use of the following medicines may enhance the vasoconstrictive effect and increase blood pressure (see also "When not to use Starazolin redFREE"):
- MAOIs used to treat Parkinson's disease or depression, e.g., selegiline, rasagiline, moclobemide, tranylcypromine,
- tricyclic antidepressants, e.g., amitriptyline and maprotiline,
- other medicines that may increase blood pressure.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Due to possible systemic side effects, the use of Starazolin redFREE in pregnant and breastfeeding women is only possible after careful consideration by the doctor of the risks and benefits associated with treatment.
There are no data available on the passage of tetryzoline hydrochloride through the placental barrier and into breast milk.
The patient should avoid using the medicine during pregnancy and breastfeeding due to possible systemic side effects.
Driving and using machines
Administration of Starazolin redFREE may cause transient, blurred vision. In such cases, the patient should not drive or operate machinery.
3. How to use Starazolin redFREE
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist. In case of doubt, the patient should consult their doctor or pharmacist.
Children under 6 years of age
Do not use in children under 2 years of age. In children between 2 and 6 years of age, the use of the medicine should be under medical supervision.
Adults and children over 6 years of age
Unless the doctor has prescribed otherwise, the patient should instill 1 to 2 drops into the affected eye, up to 3 times a day. Before instillation, the patient should remove their contact lenses and wait at least 15 minutes after administration of the medicine before putting them back in (see below - Information for contact lens wearers).
The use of the medicine in children under 12 years of age should be under adult supervision.
The medicine should not be used for more than 2 days. Use for more than 2 days should only be under medical supervision.
Method of administration
Eye drop.
Do not swallow.
Information for contact lens wearers
The patient should not wear contact lenses in case of eye disease. In special cases where contact lens wear is allowed, the patient should remove their lenses before using this medicine. After instillation of the medicine, the patient should wait 15 minutes before putting their contact lenses back in.
Instructions for use:
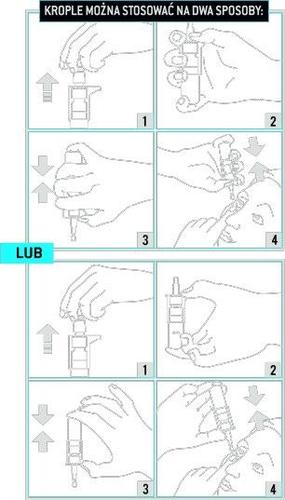
- 1. Wash your hands.
- 2. Remove the protective cap from the bottle (drawing 1).
- 3. Hold the bottle in your hand (drawing 2).
- 4. Turn the bottle upside down and press the pump until the first drop appears (drawing 3).
Then discard at least 5 first drops before instilling the medicine into the eye for the first time. Before instilling each subsequent drop, discard at least 2 drops. If the medicine has not been used for 15 days or longer, discard 5 drops before administering the medicine to the eye.
Discard at least 2 drops before instilling each subsequent drop. If the medicine has not been used for 15 days or longer, discard 5 drops before administering the medicine to the eye.
- 5. Tilt your head back and gently pull the lower eyelid down to create a pocket between it and the eye. Holding the bottle upside down, press the pump and instill one drop into the eye (drawing 4).
Do not touch the tip of the dispenser to any surfaces to avoid contaminating the solution.
- 6. Immediately after instilling the drop, press the corner of the eye near the nose or close the eyelids for 1-2 minutes. This will help prevent the medicine from getting into other parts of the body.
- 7. If the medicine is also used in the other eye, repeat steps 5 and 6.
- 8. Immediately after use, close the bottle with the protective cap.
Using more than the recommended dose of Starazolin redFREE
In case of overdose or ingestion of the medicine, the patient should immediately consult a doctor, pharmacist, or the nearest hospital and take the medicine or this patient leaflet with them.
Symptoms of local or systemic overdose of the medicine include: mydriasis, cyanosis, fever, seizures, tachycardia, arrhythmias, cardiac arrest, hypertension, pulmonary edema, respiratory disorders, and psychiatric disorders.
The risk of overdose symptoms is particularly high in infants and young children due to ingestion of the medicine. There may be central nervous system disorders, circulatory failure, and respiratory failure.
The medicine should be kept out of the reach of children. In case of ingestion, the patient should immediately seek medical help.
Missing a dose of Starazolin redFREE
The patient should not use a double dose to make up for a missed dose.
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common(occurring in less than 1 in 10 patients)
- increased conjunctival edema (reactive hyperemia), burning or dryness of the conjunctiva, palpitations, headache, tremors, weakness, sweating, increased blood pressure, rapid heart rate.
Uncommon(occurring in less than 1 in 1,000 patients)
- mydriasis.
Rare(occurring in less than 1 in 10,000 patients)
- keratinization (cornification) of the conjunctival surface, leading to closure of the tear ducts and tearing due to disorders of tear outflow after prolonged use of this medicine.
Frequency not known(frequency cannot be estimated from the available data)
- eye or periorbital burning, redness, irritation, edema, pain, itching, blurred vision, conjunctival irritation.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, it is possible to gather more information on the safety of the medicine.
5. How to store Starazolin redFREE
The medicine should be stored out of sight and reach of children.
Store in a temperature below 30°C.
Shelf life after first opening the bottle: 6 months. Store after first opening in a temperature below 30°C.
Do not use this medicine after the expiry date stated on the packaging after "EXP".
The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Starazolin redFREE contains
- The active substance of the medicine is tetryzoline hydrochloride. Each ml of solution contains 0.5 mg of tetryzoline hydrochloride.
- The other ingredients are: sodium chloride, boric acid, borax, purified water.
What Starazolin redFREE looks like and contents of the pack
Clear, colorless solution. Does not contain preservatives.
HDPE bottle with a capacity of 10 ml, with a 3K dosing pump (PP, HDPE, LDPE), protective cap made of HDPE, and applicator made of PP, in a cardboard box.
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
JADRAN-GALENSKI LABORATORIJ d.d.
Svilno 20, 51000 Rijeka, Croatia
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Starazolin redfreeDosage form: Drops, 0.5 mg/mLActive substance: tetryzolineManufacturer: Jadran-Galenski laboratorij d.d.Prescription not requiredDosage form: Drops, 0.5 mg/mlActive substance: tetryzolineManufacturer: Jadran-Galenski laboratorij d.d.Prescription not requiredDosage form: Drops, 0.5 mg/mlActive substance: tetryzolineManufacturer: Janssen Pharmaceutica N.V.Prescription not required
Alternatives to Starazolin redfree in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Starazolin redfree in Ukraine
Alternative to Starazolin redfree in Spain
Online doctors for Starazolin redfree
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Starazolin redfree – subject to medical assessment and local rules.














