
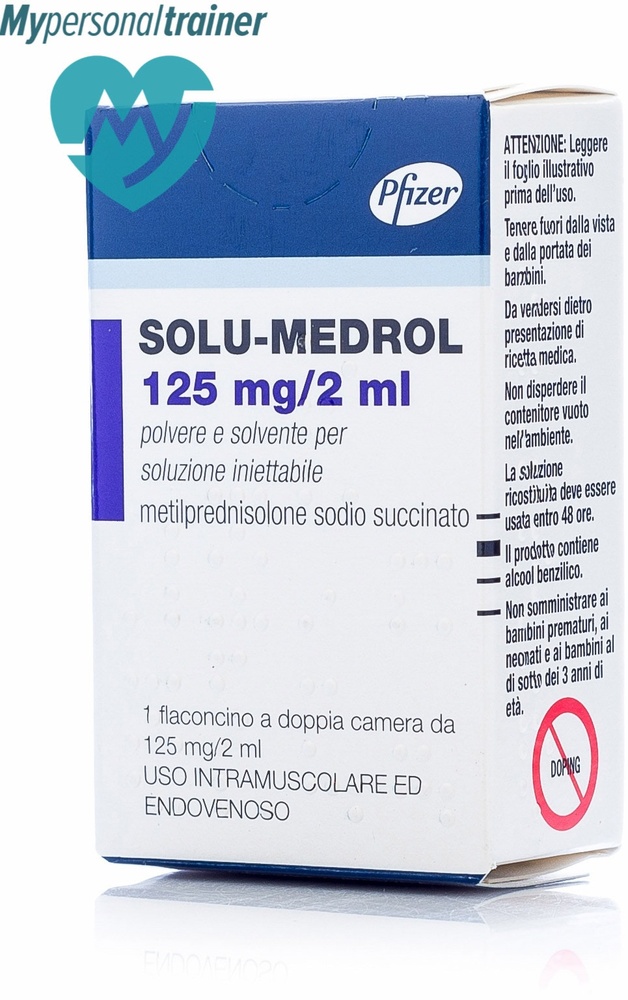
Solu-medrol

Ask a doctor about a prescription for Solu-medrol

How to use Solu-medrol
Leaflet accompanying the packaging: information for the user
SOLU-MEDROL, 40 mg, powder and solvent for solution for injection
SOLU-MEDROL, 125 mg, powder and solvent for solution for injection
SOLU-MEDROL, 500 mg, powder and solvent for solution for injection
SOLU-MEDROL, 1000 mg, powder and solvent for solution for injection
Methylprednisolone
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is SOLU-MEDROL and what is it used for
- 2. Important information before using SOLU-MEDROL
- 3. How to use SOLU-MEDROL
- 4. Possible side effects
- 5. How to store SOLU-MEDROL
- 6. Contents of the packaging and other information
1. What is SOLU-MEDROL and what is it used for
The active substance of SOLU-MEDROL, methylprednisolone, belongs to a group of medicines called corticosteroids. Corticosteroids penetrate cell membranes and bind to specific receptors located in the cytoplasm. These complexes then enter the cell nucleus, stimulating further synthesis of various enzymes that are probably responsible for the numerous actions of corticosteroids observed after general use. In addition to their significant effect on inflammatory and immune processes, corticosteroids also affect carbohydrate, protein, and fat metabolism. They also act on the cardiovascular system, skeletal muscles, and the central nervous system.
SOLU-MEDROL is used as symptomatic treatment, except in cases of endocrine disorders, when it is used as substitution therapy, in the following diseases:
Endocrine disorders
- primary or secondary adrenal cortex insufficiency (in certain circumstances, in combination with mineralocorticosteroids)
- acute adrenal cortex insufficiency (mineralocorticosteroids may need to be administered in combination)
- treatment of shock caused by adrenal cortex insufficiency, or shock that does not respond to conventional treatment, in cases where mineralocorticosteroids are contraindicated
- before surgical procedures and in cases of severe illness or injury, in patients with diagnosed adrenal cortex insufficiency or decreased adrenal hormone levels
- congenital adrenal hyperplasia
- non-suppurative thyroiditis
- hypercalcemia in cancer
Rheumatic diseases
Supportive treatment for short-term use during episodes of exacerbation or worsening of the condition in:
post-traumatic degenerative joint disease
synovial membrane inflammation in degenerative joint disease
rheumatoid arthritis, including juvenile rheumatoid arthritis
acute and subacute bursitis
epicondylitis
acute non-specific tenosynovitis
acute gouty arthritis
psoriatic arthritis
ankylosing spondylitis
Systemic connective tissue diseases
During exacerbation or as maintenance therapy in:
systemic lupus erythematosus (and kidney inflammation in lupus)
acute rheumatic myocarditis
systemic polymyositis and dermatomyositis
giant cell arteritis
Goodpasture's syndrome
Dermatological diseases
pemphigus
severe form of erythema multiforme (Stevens-Johnson syndrome)
exfoliative dermatitis
severe form of psoriasis
bullous pemphigoid
severe form of seborrheic dermatitis
mycosis fungoides
Allergic diseases
Treatment of severe allergic diseases, when other treatment methods are ineffective:
bronchial asthma
contact dermatitis (contact eczema)
atopic dermatitis
chickenpox
drug hypersensitivity reaction
transfusion-related urticaria
acute non-inflammatory laryngeal edema (epinephrine is the first-choice drug)
Ophthalmic diseases
Severe, acute, and chronic allergic and inflammatory processes involving the eye and its appendages, such as:
ophthalmic zoster
uveitis, uveitis and corpus vitreum inflammation
retinal and choroid inflammation
diffuse choroiditis and posterior segment inflammation
optic neuritis
sympathetic ophthalmia
inflammation of the anterior segment of the eye
allergic conjunctivitis
allergic marginal corneal ulcers
keratitis
Gastrointestinal diseases
As systemic therapy in exacerbation of:
ulcerative colitis
Crohn's disease
Respiratory diseases
Symptomatic sarcoidosis
Berylliosis
Fulminant or disseminated pulmonary tuberculosis, in combination with appropriate antitubercular chemotherapy
Loeffler's syndrome not responding to other treatments
Aspiration pneumonia
Moderate or severe pneumonia caused by Pneumocystis jiroveci in patients with AIDS (as supportive treatment, when administered within the first 72 hours of initial treatment against Pneumocystis)
Hematological diseases
Acquired (autoimmune) hemolytic anemia
Idiopathic thrombocytopenic purpura in adults (only intravenous administration; intramuscular administration is contraindicated)
Secondary thrombocytopenia in adults
Erythroblastopenia in the bone marrow
Congenital hypoplastic anemia
Oncological diseases
Palliative treatment:
leukemia and lymphoma in adults
acute leukemia in children
improving the quality of life of patients with advanced cancer
Edema
To induce diuresis or remission of proteinuria in nephrotic syndrome, without azotemia
Nervous system
Brain edema associated with the presence of a primary or metastatic tumor, and (or) related to surgical treatment or radiation therapy
Exacerbation in multiple sclerosis
Acute spinal cord injuries. Treatment should be started within eight hours of injury.
Other indications
Tuberculous meningitis with subarachnoid block or risk of subarachnoid block, along with appropriate antitubercular therapy
Trichinosis with involvement of the nervous system or heart muscle
Organ transplantation
Prevention of nausea and vomiting associated with cancer chemotherapy
2. Important information before using SOLU-MEDROL
When not to use SOLU-MEDROL
- for intrathecal administration
- for epidural administration
- in premature infants and newborns
Administration of live or live attenuated vaccines is contraindicated during treatment with SOLU-MEDROL, at doses that cause immunosuppressive effects.
Warnings and precautions
In patients with the following diseases, treatment with SOLU-MEDROL should be as short as possible and they require special medical care during treatment with SOLU-MEDROL.
Before starting to take this medicine, the patient should discuss it with their doctor or pharmacist if they have any of the following conditions:
Infectious diseases, such as tuberculosis and certain viral diseases (herpes and zoster with eye symptoms).
The use of SOLU-MEDROL in active tuberculosis should be limited to cases of fulminant or disseminated pulmonary tuberculosis, in which SOLU-MEDROL is used in combination with antitubercular chemotherapy. During long-term treatment with SOLU-MEDROL, in patients with latent tuberculosis or a positive tuberculin test, careful observation is necessary, as the disease may recur.
In patients with ocular herpes simplex infection, who are treated with SOLU-MEDROL, there is a risk of corneal perforation.
Diabetes
Treatment with SOLU-MEDROL may cause symptoms of latent diabetes, increased insulin requirements, or oral hypoglycemic agents.
Hypertension
Treatment with SOLU-MEDROL may exacerbate hypertension.
Psychiatric disordersoccurring currently or in the past
SOLU-MEDROL may exacerbate existing emotional instability or psychotic tendencies. During treatment, psychiatric disorders may occur, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression to severe psychotic disorders.
Severe stress
In patients exposed to severe stress, it may be necessary to increase the dose of rapidly acting corticosteroids before, during, and after the stress-causing situation.
Allergyto any medicines
If the patient has had an allergic reaction to any medicine, they should inform their doctor before starting treatment with SOLU-MEDROL.
Hypothyroidism
The effect of SOLU-MEDROL is stronger in patients with hypothyroidism.
Hyperthyroidism
Liver cirrhosis
The effect of SOLU-MEDROL is stronger in patients with liver cirrhosis.
Head injury
Nonspecific ulcerative colitis, in case of possible perforation, abscess, or other purulent infection
Diverticulitis
Recent intestinal anastomosis
Active or latent peptic ulcer
Kidney failure
Osteoporosis
Myasthenia gravis(acquired, chronic disease characterized by rapid fatigue and muscle weakness).
If the patient experiences blurred vision or other visual disturbances, they should contact their doctor.
Immunosuppressive effect, increased susceptibility to infections
SOLU-MEDROL may increase the susceptibility to infections and may mask some symptoms of infection. During its use, new infections may develop. During the use of SOLU-MEDROL, there may be decreased immunity and inability to limit the development of infection. The risk of infections with pathogens such as viruses, bacteria, fungi, protozoa, or parasites increases with increasing dose.
Patients using SOLU-MEDROL are more susceptible to infections than healthy individuals, e.g., in children with immunodeficiency or in adults using SOLU-MEDROL, chickenpox and measles can have a more severe course or even be fatal.
During treatment with SOLU-MEDROL, at doses that cause immunosuppressive effects, it is contraindicated to administer certain vaccines. Inactivated vaccines may be administered, but the response to them may be limited or they may be ineffective. Patients receiving SOLU-MEDROL at doses that do not have immunosuppressive effects may be subjected to all required vaccinations.
Kaposi's sarcoma has been observed in patients treated with SOLU-MEDROL. Discontinuation of treatment may lead to remission of the disease.
Effect on the immune system
Patients may experience allergic reactions. In patients taking SOLU-MEDROL, skin reactions and anaphylactic/pseudoanaphylactic reactions have occurred rarely.
Endocrine disorders
During long-term treatment with SOLU-MEDROL, adrenal insufficiency may occur, which may persist for several months after discontinuation of treatment.
The doctor may also decide to gradually reduce the dose of SOLU-MEDROL. The patient should inform their doctor about any stressful situations that occur during this period. The doctor will consider initiating hormone therapy.
Sudden discontinuation of SOLU-MEDROL may cause acute adrenal cortex insufficiency, leading to death.
After sudden discontinuation of SOLU-MEDROL, a "steroid withdrawal syndrome" may also occur. This syndrome includes symptoms such as loss of appetite, nausea, vomiting, lethargy, headache, fever, joint pain, peeling, muscle pain, weight loss, and (or) hypotension.
SOLU-MEDROL may cause or exacerbate Cushing's syndrome, so patients with Cushing's disease should not use it.
In patients with hypothyroidism, the effect of SOLU-MEDROL is stronger.
The patient should immediately consult their doctor if they experience weakness or muscle pain, cramps, and stiffness while taking methylprednisolone. These may be symptoms of a condition called thyrotoxic periodic paralysis, which can occur in patients with hyperthyroidism treated with methylprednisolone. Additional treatment may be necessary to alleviate this condition.
Psychiatric disorders
During treatment with SOLU-MEDROL and after its completion, psychiatric disorders may occur. They usually occur within a few days or weeks of starting treatment with SOLU-MEDROL. Most of them disappear after reducing the dose or discontinuing SOLU-MEDROL. Patients and their caregivers should consult their doctor if the patient develops psychological symptoms, especially if they suspect depressive mood or suicidal thoughts. Patients and their caregivers should pay special attention to psychiatric disorders that may occur during treatment, immediately after dose reduction, or after discontinuation of SOLU-MEDROL.
Effect on the nervous system
SOLU-MEDROL should be used with caution in patients with seizure disorders.
In patients using SOLU-MEDROL, usually with long-term use of high doses, cases of supratentorial lipoma have been reported.
Effect on the eye
In patients using SOLU-MEDROL for a long time, posterior subcapsular cataract and nuclear cataract (especially in children) may develop, as well as exophthalmos or increased intraocular pressure, which can cause glaucoma with potential optic nerve damage.
Patients using SOLU-MEDROL may also be more likely to develop secondary fungal or viral eye infections.
As a result of systemic and local use of the medicine, visual disturbances may occur. If they occur, the patient should contact their doctor to determine possible causes, which may include cataract, glaucoma, or rare diseases such as central serous chorioretinopathy.
Central serous chorioretinopathy can lead to retinal detachment.
The use of SOLU-MEDROL is associated with the occurrence of central serous chorioretinopathy, which can lead to retinal detachment.
Effect on the heart
In the case of high-dose and long-term treatment with SOLU-MEDROL, in patients with cardiovascular risk factors, it should be administered with caution and with additional monitoring of the cardiovascular system if necessary. The frequency of complications associated with the use of SOLU-MEDROL can be reduced by using small doses and administering the medicine every other day.
After rapid intravenous administration of high doses of SOLU-MEDROL, cardiac arrhythmias and (or) circulatory collapse, and (or) cardiac arrest may occur.
In patients with congestive heart failure, SOLU-MEDROL should be administered with caution and only if necessary.
Effect on the vascular system
During the use of SOLU-MEDROL, thrombosis, including venous thromboembolism, has been reported. Therefore, caution should be exercised in patients with thromboembolic disorders or those who may be prone to them.
Effect on the stomach and intestines
After the use of high doses of SOLU-MEDROL, acute pancreatitis may occur.
Treatment with SOLU-MEDROL may mask symptoms of peptic ulcers, so perforation or bleeding may occur without significant pain. Corticosteroid treatment may mask peritonitis or other symptoms related to gastrointestinal disorders, such as perforation, obstruction, or pancreatitis.
The combination of SOLU-MEDROL with non-steroidal anti-inflammatory drugs (NSAIDs) increases the risk of developing peptic ulcer disease and gastrointestinal bleeding.
Effect on the liver and bile ducts
Cyclic, pulse intravenous administration of methylprednisolone may cause drug-induced liver damage, such as acute hepatitis. Acute hepatitis may occur within a few weeks or longer. After discontinuation of treatment, this adverse event has been observed to resolve.
Effect on the musculoskeletal system
During the use of high doses of SOLU-MEDROL, acute myopathy may occur, especially in patients with neuromuscular transmission disorders (e.g., myasthenia gravis) or in patients treated with anticholinergic drugs, including neuromuscular blockers (e.g., pancuronium). Myopathy may affect the eye muscles and respiratory muscles, leading to quadriparesis. Elevated creatine kinase activity may occur. Improvement in clinical condition or complete recovery may occur after discontinuation of SOLU-MEDROL, after several weeks or even years.
Effect on the kidneys and urinary tract
Caution should be exercised in patients with systemic sclerosis, as an increased frequency of scleroderma renal crisis has been observed with corticosteroids, including methylprednisolone.
SOLU-MEDROL should be used with caution in patients with renal failure.
Diagnostic tests
In patients using medium to high doses of SOLU-MEDROL, blood pressure, sodium and water retention, and potassium excretion may increase. Therefore, it may be necessary to restrict salt in the diet and supplement potassium. All corticosteroids, including SOLU-MEDROL, increase calcium excretion.
Injuries, poisonings, and post-procedure complications
SOLU-MEDROL should not be used to treat traumatic brain injury.
Other
The complications of corticosteroid therapy depend on the dose and duration of treatment. The doctor will decide on the dosage and duration of treatment individually for each patient.
Patients should be cautious when using aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) with SOLU-MEDROL.
After administration of SOLU-MEDROL, a breakthrough in the course of a pheochromocytoma has been reported, sometimes fatal. In patients suspected of or diagnosed with pheochromocytoma, the doctor will decide on the use of SOLU-MEDROL only after careful assessment of the benefit-to-risk ratio.
Tumor lysis syndrome may occur during corticosteroid treatment for cancer. The patient should inform their doctor if they have cancer and experience symptoms of tumor lysis syndrome, such as muscle cramps, weakness, confusion, irregular heartbeat, vision loss, or shortness of breath.
SOLU-MEDROL and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. SOLU-MEDROL may affect the action of other medicines, and other medicines may affect the action of SOLU-MEDROL.
It may be necessary to adjust the dose of SOLU-MEDROL when used in combination with the following medicines:
antibacterial drugs: isoniazid
antitubercular antibiotic: rifampicin
- anticoagulant drugs (oral). Concomitant use with SOLU-MEDROL may decrease or increase the effect of anticoagulant drugs. Blood coagulation tests should be monitored to ensure adequate anticoagulant effect.
- anticonvulsant drugs: carbamazepine, phenobarbital, phenytoin
- anticholinergic drugs: neuromuscular blockers. During concomitant use of high doses of SOLU-MEDROL and anticholinergic drugs, such as neuromuscular blockers, cases of acute myopathy have been reported.
- muscle relaxants, e.g., pancuronium, vecuronium: SOLU-MEDROL may partially inhibit neuromuscular blockade caused by muscle relaxants
- anticholinesterases: SOLU-MEDROL may reduce the effect of anticholinesterases in patients with myasthenia gravis
anti-diabetic drugs: in diabetic patients, it may be necessary to adjust the dose of anti-diabetic drugs, as SOLU-MEDROL may increase blood glucose levels
anti-emetic drugs: aprepitant, fosaprepitant
antifungal drugs: itraconazole, ketoconazole
antiviral drugs - HIV protease inhibitors: indinavir and ritonavir
aromatase inhibitor: aminoglutethimide
calcium channel blocker: diltiazem
oral contraceptives: ethinyl estradiol/norethindrone
grapefruit juice
immunosuppressive drugs: cyclosporine. When cyclosporine and SOLU-MEDROL are used concomitantly, there is mutual inhibition of metabolism, which may increase the plasma concentration of one or both drugs. Therefore, there is a possibility that the risk of adverse effects associated with the use of one of the drugs may increase during concomitant administration. Cases of seizures have been reported during concomitant use.
immunosuppressive drugs: cyclophosphamide, tacrolimus
macrolide antibacterial drugs: clarithromycin, erythromycin, troleandomycin
- non-steroidal anti-inflammatory drugs (NSAIDs): high doses of aspirin. Concomitant use of NSAIDs with SOLU-MEDROL may increase the frequency of gastrointestinal bleeding and ulceration. Caution should be exercised when using aspirin in combination with SOLU-MEDROL
- drugs that reduce potassium levels. During concomitant use of SOLU-MEDROL with drugs that reduce potassium levels (e.g., diuretics), patients should be monitored for the development of hypokalemia (a condition in which the potassium ion concentration in the blood is below the laboratory norm). During concomitant use of SOLU-MEDROL with amphotericin B, xanthines, or beta2 agonists, the risk of hypokalemia increases.
Pregnancy, breastfeeding, and fertility
Fertility
In animal studies, it has been shown that SOLU-MEDROL reduces fertility.
Pregnancy
If the patient is pregnant or breastfeeding, or thinks they may be pregnant or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
In some animal studies, it has been shown that corticosteroids administered to pregnant mothers in high doses may cause fetal developmental abnormalities. However, it does not appear that SOLU-MEDROL administered to pregnant women causes congenital abnormalities in the fetus. Until appropriate studies are conducted on the effect of SOLU-MEDROL on human reproductive processes, this medicine should not be administered to pregnant women unless a thorough assessment of the benefit-to-risk ratio for the mother and fetus is made.
SOLU-MEDROL crosses the placenta. In one retrospective study, an increased frequency of low birth weight in newborns born to mothers who took corticosteroids was found. In humans, the risk of low birth weight is dose-dependent. This risk can be reduced by administering lower doses of corticosteroids.
Although neonatal adrenal insufficiency is rare in infants exposed to SOLU-MEDROL in utero, children born to mothers who took high doses of SOLU-MEDROL during pregnancy should be carefully monitored and evaluated for adrenal insufficiency.
The effect of SOLU-MEDROL on the course of labor is not known.
In infants born to mothers treated with SOLU-MEDROL for a long time during pregnancy, cataract development has been observed.
SOLU-MEDROL 500 mg and 1000 mg, powder and solvent for solution for injection, contains benzyl alcohol (see section 2 "SOLU-MEDROL 500 mg and 1000 mg contains benzyl alcohol").
Breastfeeding
SOLU-MEDROL is excreted in human milk.
In breastfed children, SOLU-MEDROL that has passed into the mother's milk may inhibit growth and affect the production of endogenous corticosteroids. This medicine may be used by breastfeeding women only after careful assessment of the benefit-to-risk ratio for the mother and infant.
SOLU-MEDROL 500 mg and 1000 mg, powder and solvent for solution for injection, contains benzyl alcohol (see section 2 "SOLU-MEDROL 500 mg and 1000 mg contains benzyl alcohol").
Driving and using machines
The effect of SOLU-MEDROL on the ability to drive and use machines has not been studied.
Patients who experience dizziness, visual disturbances, and fatigue while using SOLU-MEDROL should not drive or operate machinery.
SOLU-MEDROL 500 mg and 1000 mg contains benzyl alcohol
SOLU-MEDROL, 500 mg and 1000 mg, in the form of powder and solvent for solution for injection, contains 9 mg of benzyl alcohol in each 1 ml of solution, which corresponds to 9 mg/1 ml of benzyl alcohol. Benzyl alcohol may cause allergic reactions. Administration of benzyl alcohol to newborns and young children is associated with a risk of serious adverse effects, including respiratory disorders. SOLU-MEDROL containing benzyl alcohol should not be used in newborns (up to 4 weeks of age) and should not be used in young children (under 3 years of age) for more than a week without a doctor's recommendation. The patient should consult their doctor or pharmacist if they have liver or kidney disease, or if they are pregnant or breastfeeding, as a large amount of benzyl alcohol may accumulate in the body and cause adverse effects such as increased blood acidity (metabolic acidosis).
SOLU-MEDROL contains sodium
SOLU-MEDROL, 40 mg and 125 mg, powder and solvent for solution for injection, contains less than 1 mmol (23 mg) of sodium per vial, which means the medicine is considered "sodium-free".
SOLU-MEDROL, 500 mg, powder and solvent for solution for injection, contains 58.3 mg of sodium (the main component of table salt) per vial. This corresponds to 2.92% of the maximum recommended daily sodium intake in the diet for adults.
SOLU-MEDROL, 1000 mg, powder and solvent for solution for injection, contains 116.8 mg of sodium (the main component of table salt) per vial. This corresponds to 5.84% of the maximum recommended daily sodium intake in the diet for adults.
3. How to use SOLU-MEDROL
This medicine should be used as directed by the doctor, who will adjust the dose individually for each patient. If there are any doubts, the patient should consult their doctor.
During treatment with SOLU-MEDROL, as well as after discontinuation of long-term treatment, the patient should remain under close medical supervision.
SOLU-MEDROL can be administered by intravenous or intramuscular injection, or by intravenous infusion. The dose may be reduced in infants and children, but should be based on the patient's condition and response to treatment, rather than age or body weight (it should not be less than 0.5 mg/kg body weight/24 hours).
Dosing requirements are variable and should be individualized, depending on the disease being treated, its severity, and the patient's response to treatment throughout the treatment period. The decision based on the benefit-to-risk ratio in each individual case should be made on an ongoing basis.
It is recommended to use the smallest effective dose of corticosteroid that will provide control of the treated disease, for the shortest possible time. The appropriate maintenance dose should be determined by gradually reducing the initial dose of SOLU-MEDROL in appropriate time intervals until the smallest dose that will provide an adequate clinical response is reached.
If treatment is to be discontinued after a long period of use, SOLU-MEDROL should be gradually discontinued; it should not be stopped abruptly.
After the initial period of use in emergency situations, it may be necessary to switch to treatment with a long-acting injection or oral medicine.
If the medicine is used as supportive treatment in life-threatening conditions, it should be administered intravenously in a dose of 30 mg/kg body weight over at least 30 minutes. The dose can be repeated every 4 to 6 hours for a period not exceeding 48 hours.
Administration of methylprednisolone in the form of intravenous pulses in a dose of 250 mg/day or higher for several days (usually ≤ 5 days) may be effective in the treatment of exacerbations of disease or conditions for which standard therapy is ineffective. These include rheumatic diseases, systemic lupus erythematosus, and edematous conditions such as nephrotic syndrome or lupus nephritis. In patients with multiple sclerosis, who have not responded to standard therapy (or in patients with exacerbations of the disease), 30-minute intravenous pulses in doses of 500 mg/day or 1000 mg/day should be administered for 3 or 5 days.
If the medicine is used as supportive treatment for other conditions, the initial intravenous dose will vary from 10 to 500 mg, depending on the patient's clinical condition.
In the case of short-term treatment of severe, acute conditions, higher doses may be required. Initial doses not exceeding 250 mg should be administered intravenously over at least 5 minutes, while higher doses should be administered over at least 30 minutes.
Subsequent doses can be administered intravenously or intramuscularly at intervals dictated by the patient's response to treatment and clinical condition.
During prolonged therapy, routine laboratory tests should be performed regularly, such as urinalysis, postprandial glucose levels, blood pressure measurement, body weight measurement, and chest X-ray. Radiological images of the upper gastrointestinal tract are required in patients with a history of ulcers or significant dyspepsia.
If the patient feels that the effect of SOLU-MEDROL is too strong or too weak, they should consult their doctor.
Use of a higher than recommended dose of SOLU-MEDROL
In case of overdose, the patient should immediately consult their doctor or pharmacist. There are no clinical symptoms of acute overdose of SOLU-MEDROL. Chronic overdose causes typical symptoms of Cushing's syndrome. Dialysis is an effective method of removing SOLU-MEDROL from the body.
Missing a dose of SOLU-MEDROL
The patient should not take a double dose to make up for a missed dose.
Discontinuation of SOLU-MEDROL
In case of any further doubts related to the use of this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, SOLU-MEDROL can cause side effects, although not everybody gets them.
The following side effects have been reported for the following routes of administration:
intrathecal/epidural: meningitis, gastrointestinal or urinary tract disorders, headache, meningitis, transverse myelitis, or seizures.
If the patient experiences any of the following symptoms, they should immediately inform their doctor or go to the nearest hospital:
5. How to store SOLU-MEDROL
SOLU-MEDROL, 40 mg, 125 mg, 500 mg, 1000 mg:
No special storage precautions are required for the medicinal product.
SOLU-MEDROL, 40 mg:
After reconstitution, the product should be stored at a temperature below 25°C and used immediately or stored at a temperature of 2°C - 8°C and used within 48 hours.
After reconstitution and further dilution with other infusion solutions, the product should be stored at a temperature of 20°C - 25°C and used within 3 hours or stored at a temperature of 2°C - 8°C and used within 24 hours.
SOLU-MEDROL, 125 mg, 500 mg, 1000 mg:
After reconstitution, the product should be stored at a temperature below 25°C and used within 12 hours or stored at a temperature of 2°C - 8°C and used within 48 hours.
After reconstitution and further dilution with other infusion solutions, the product should be stored at a temperature of 20°C - 25°C and used within 3 hours or stored at a temperature of 2°C - 8°C and used within 24 hours.
From a microbiological point of view, the solution should be used immediately, unless the product has been opened and diluted under controlled and validated aseptic conditions.
If the prepared solution is not used immediately, the user is responsible for the storage conditions and storage time.
More information on the storage of reconstituted and diluted solutions can be found in the section: "Information intended for healthcare professionals only".
The medicinal product should be stored out of the sight and reach of children.
Do not use this medicinal product after the expiry date stated on the carton after: (EXP). The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer required. This will help protect the environment.
6. Package contents and other information
What SOLU-MEDROL contains
SOLU-MEDROL, 40 mg, powder and solvent for solution for injection
- The active substance is methylprednisolone in the form of sodium succinate.
- The other excipients are: sucrose, sodium dihydrogen phosphate monohydrate, disodium phosphate anhydrous (see section 2 "SOLU-MEDROL contains sodium"). Solvent: water for injections.
SOLU-MEDROL, 125 mg, powder and solvent for solution for injection
- The active substance is methylprednisolone in the form of sodium succinate.
- The other excipients are: sodium dihydrogen phosphate monohydrate, disodium phosphate anhydrous (see section 2 "SOLU-MEDROL contains sodium"). Solvent: water for injections.
SOLU-MEDROL, 500 mg, 1000 mg, powder and solvent for solution for injection
- The active substance is methylprednisolone in the form of sodium succinate.
- The other excipients are: sodium dihydrogen phosphate monohydrate, disodium phosphate anhydrous (see section 2 "SOLU-MEDROL contains sodium"). Solvent: benzyl alcohol (E1519) (see section 2 "SOLU-MEDROL 500 mg and 1000 mg contain benzyl alcohol"), water for injections.
What SOLU-MEDROL looks like and contents of the pack
SOLU-MEDROL is a white, compact powder and a clear, colorless solvent.
Packaging contents:
SOLU-MEDROL, 40 mg
A two-chamber vial made of colorless glass with powder and solvent of 1 ml in a cardboard box.
SOLU-MEDROL, 125 mg
A two-chamber vial made of colorless glass with powder and solvent of 2 ml in a cardboard box.
SOLU-MEDROL, 500 mg
A vial made of colorless glass with powder and a vial with solvent of 8 ml in a cardboard box.
SOLU-MEDROL, 1000 mg
A vial made of colorless glass with powder and a vial with solvent of 16 ml in a cardboard box.
Marketing authorization holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturer
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Belgium
To obtain more detailed information, please contact the local representative of the marketing authorization holder:
Pfizer Polska Sp. z o.o.
tel. 22 335 61 00
Date of last revision of the leaflet:
---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only
Incompatibilities
It is recommended to administer methylprednisolone sodium succinate separately, without other intravenously administered compounds, to avoid problems with compatibility or stability. Medicines that are not compatible in terms of physical parameters in a solution with methylprednisolone sodium succinate include, but are not limited to: allopurinol sodium, doksapram hydrochloride, tigecycline, diltiazem hydrochloride, calcium gluconate, vecuronium bromide, rocuronium bromide, cisatracurium besylate, glycopyrrolate, propofol.
The compatibility and stability of intravenously administered methylprednisolone sodium succinate solution and in combination with other products depend on the pH of the solution, concentration, time, temperature, and degree of solubility of methylprednisolone in the given solution. Therefore, whenever possible, the methylprednisolone sodium succinate solution should be administered separately, in the form of a bolus or intravenous infusion, or "piggy-back" infusion.
To administer SOLU-MEDROL in the form of a bolus or intravenous infusion, or "piggy-back" infusion, the solution should be prepared according to the recommendations.
PREPARATION OF THE SOLUTION
Under aseptic conditions, add the solvent to the vial containing the sterile powder. Use only the specially designed solvent. Treatment can be started by administering the methylprednisolone sodium succinate solution intravenously over at least 5 minutes (doses up to 250 mg) or over at least 30 minutes (doses of 250 mg and above). Subsequent doses can be administered in a similar manner.
SOLU-MEDROL, 40 mg:
The reconstituted solution should be stored at a temperature of 2°C - 8°C and used within 48 hours. If stored at a temperature below 25°C, it should be used immediately.
SOLU-MEDROL, 125 mg, 500 mg, 1000 mg:
The reconstituted solution should be stored at a temperature below 25°C and used within 12 hours or stored at a temperature of 2°C - 8°C and used within 48 hours.
If necessary, the reconstituted solution can be further diluted in solutions:
- 5% dextrose solution in water for injections
- physiological saline solution
- 5% dextrose solution in 0.45% NaCl solution
- 5% dextrose solution in 0.9% NaCl solution
SOLU-MEDROL, 40 mg:
The reconstituted and further diluted solution stored at a temperature of 2°C - 8°C is chemically and physically stable for 24 hours. If stored at a temperature of 20°C - 25°C, it should be used within 3 hours.
SOLU-MEDROL, 125 mg, 500 mg, 1000 mg:
The reconstituted and further diluted solution stored at a temperature of 20°C - 25°C is chemically and physically stable for 3 hours or 24 hours when stored at a temperature of 2°C - 8°C.
From a microbiological point of view, the solution should be used immediately, unless the product has been opened and diluted under controlled and validated aseptic conditions.
If the prepared solution is not used immediately, the user is responsible for the storage conditions and storage time.
INSTRUCTIONS FOR PREPARING THE SOLUTION IN TWO-CHAMBER VIALS (SOLU-MEDROL, 40 mg and SOLU-MEDROL, 125 mg)
- 1. Press the plastic activator to transfer the solvent to the lower chamber.
- 2. Gently shake to obtain a solution.
- 3. Remove the plastic cap covering the center of the stopper.
- 4. Sterilize the top of the stopper with a suitable bactericidal agent.
Note: Steps 1 to 4 should be performed before withdrawing the medicinal product.
- 5. Insert the needle perpendicularly through the center of the stopperuntil the tip is visible.
- 6. Turn the vial over and withdraw the required dose.
Medicines administered parenterally should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterPfizer Manufacturing Belgium NV
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Solu-medrolDosage form: Suspension, (40 mg + 10 mg)/mlActive substance: methylprednisoloneManufacturer: Pfizer Manufacturing Belgium NVPrescription requiredDosage form: Suspension, 40 mg/mlActive substance: methylprednisoloneManufacturer: Pfizer Manufacturing Belgium NVPrescription requiredDosage form: Tablets, 4 mgActive substance: methylprednisoloneManufacturer: Pfizer Italia S.r.l.Prescription required
Alternatives to Solu-medrol in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Solu-medrol in Spain
Alternative to Solu-medrol in Ukraine
Online doctors for Solu-medrol
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Solu-medrol – subject to medical assessment and local rules.










