
Smoflipid

Ask a doctor about a prescription for Smoflipid

How to use Smoflipid
Leaflet attached to the packaging: information for the user
SMOFlipid, 200 mg/ml, infusion emulsion
Complex product
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
Keep this leaflet, you may need to read it again.
In case of any doubts, consult a doctor or nurse.
If the patient experiences any side effects, including those not listed in this leaflet, tell the doctor or nurse. See section 4.
Table of contents of the leaflet
- 1. What is SMOFlipid and what is it used for
- 2. Important information before using SMOFlipid
- 3. How to use SMOFlipid
- 4. Possible side effects
- 5. How to store SMOFlipid
- 6. Contents of the packaging and other information
1. What is SMOFlipid and what is it used for
SMOFlipid contains four different fats (lipids): soybean oil, medium-chain triglycerides, olive oil, and fish oil rich in omega-3 fatty acids. The liquid is a mixture of fat and water, i.e. a so-called "fat emulsion".
- The action of the medicine is to provide the body with energy and fatty acids.
- The medicine is administered into the blood through a drip or infusion pump. Medical personnel administer SMOFlipid to patients for whom other methods of nutrition are insufficient, impossible, or contraindicated.
2. Important information before using SMOFlipid
Do not use SMOFlipid if the patient has:
- allergy to soybean oil, medium-chain triglycerides, olive oil, fish oil, or any of the other ingredients of this medicine (listed in section 6);
- allergy to products containing fish, eggs, soy, or peanuts;
- excessive fat in the blood (severe hyperlipidemia);
- severe kidney or liver failure;
- severe blood coagulation disorders (coagulation disorders);
- acute shock;
- fluid in the lungs (pulmonary edema), excess water in the body (overhydration), or heart failure (caused by excess water in the body);
- unstable general condition, e.g. severe post-traumatic condition, heart attack, stroke, blood clot (embolism), metabolic acidosis (metabolic disorder causing high acid levels in the blood), or uncontrolled diabetes, severe infection (systemic inflammatory response syndrome - sepsis), and dehydration.
Warnings and precautions
Before starting to use SMOFlipid, inform the doctor or nurse if the patient has too high a level of fat in the blood due to the body's inability to properly eliminate fat (disrupted fat metabolism).
When used in newborns and children under 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration (see section 2). Exposure of SMOFlipid to light, especially after adding trace elements and (or) vitamins, leads to the formation of peroxides and other breakdown products, which can be limited by providing protection from light.
Allergic reactions
If an allergic reaction occurs during the use of SMOFlipid, discontinue the use of this medicine immediately. Inform the doctor or nurse immediately if the following symptoms occur during infusion:
- fever (high temperature);
- chills;
- rash;
- breathing difficulties.
Children
Consult a doctor or nurse if the medicine is given to a newborn who has:
- too much of a substance called "bilirubin" in the blood (hyperbilirubinemia);
- high blood pressure in the lungs (pulmonary hypertension). During long-term administration of SMOFlipid to newborns, the doctor will order blood tests to monitor the patient's health.
SMOFlipid and other medicines
Inform the doctor about all medicines currently or recently used by the patient, as well as medicines that the patient plans to use.
In particular, inform the doctor about the use of medicines that reduce blood clotting, such as warfarin and heparin.
- SMOFlipid contains vitamin K, which may affect warfarin. However, the amount of vitamin K in SMOFlipid is so small that such problems should not occur.
- Heparin administered in clinical doses may temporarily increase the level of fatty acids in the blood, which is caused by the release of fatty acids from tissues into the bloodstream, followed by a decrease in the amount of fatty acids excreted from the blood (decreased triglyceride clearance).
Pregnancy and breastfeeding
There is no data on the use of SMOFlipid in pregnant or breastfeeding women. Therefore, SMOFlipid is given to pregnant or breastfeeding women only when the doctor considers it necessary.
If the patient is pregnant or breastfeeding, suspects that she may be pregnant, or plans to have a child, she should consult a doctor or pharmacist before using this medicine.
Driving and using machines
This does not apply, as the medicine is used in a hospital.
SMOFlipid contains sodium
This medicine contains 5 mmol (115 mg) of sodium per 1000 ml. This should be taken into account in patients controlling their sodium intake.
3. How to use SMOFlipid
SMOFlipid is administered into the blood through a drip or infusion pump.
The doctor determines the individual dose based on the patient's age, weight, and ability to utilize the administered fat.
When used in newborns and children under 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration (see section 2).
Detailed information on dosing and administration can be found at the end of the leaflet in the section "Information intended exclusively for healthcare professionals".
Using a higher dose of SMOFlipid than recommended
If the patient uses a higher dose of SMOFlipid than recommended, there is a risk of taking too much fat and problems with fat metabolism. This is called "fat overload syndrome". More information can be found in section 4. Possible side effects.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Fat overload syndrome
Fat overload syndrome occurs when the body has a problem with fat metabolism due to receiving too much SMOFlipid. It can also occur due to a sudden change in the patient's health (e.g. kidney problems or infection). Possible symptoms include increased fat levels in the blood (hyperlipidemia) and in cells and tissues, fever, disorders of many organs, and coma. All these symptoms usually disappear after the infusion is stopped.
Common side effects(may occur in up to 1 in 10 patients):
- mild increase in body temperature.
Uncommon side effects(may occur in up to 1 in 100 patients):
- chills;
- loss of appetite;
- nausea;
- vomiting.
Rare side effects(may occur in up to 1 in 1000 patients):
- allergic reactions (e.g. high temperature, swelling, low blood pressure, rash, redness, headache);
- feeling of cold or heat;
- pallor;
- blue discoloration of the skin and mucous membranes (caused by low oxygen levels in the blood);
- pains in the neck, back, bones, chest, and lumbar region;
- increased or decreased blood pressure;
- shortness of breath.
Very rare side effects(may occur in up to 1 in 10,000 patients):
- prolonged and painful erection of the penis.
Reporting side effects
If any side effects occur, including those not listed in the leaflet, tell the doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products Al. Jerozolimskie 181C 02-222 Warsaw tel.: +48 22 49 21 301 fax: +48 22 49 21 309 e-mail: [email protected] Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of the medicine.
5. How to store SMOFlipid
Store in a place out of sight and reach of children.
Do not store at a temperature above 25°C. Do not freeze.
Do not use the medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Do not use the medicine SMOFlipid if the packaging is damaged. Use only if the emulsion is white and homogeneous. For single use only. Dispose of any unused residue. Do not reuse.
When used in newborns and children under 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration (see section 2).
6. Contents of the packaging and other information
What SMOFlipid contains
- The active substances of the medicine are: soybean oil, purified 60 mg/ml medium-chain triglycerides 60 mg/ml olive oil, purified 50 mg/ml fish oil, rich in omega-3 fatty acids 30 mg/ml
- Other ingredients are: egg lecithin, glycerol, sodium oleate, all-rac-α-tocopherol, sodium hydroxide, water for injections.
What SMOFlipid looks like and what the packaging contains
SMOFlipid is a white, homogeneous emulsion available in glass bottles or Biofine bags.
Packaging sizes:
- glass bottle: 1 x 100 ml, 10 x 100 ml, 1 x 250 ml, 10 x 250 ml, 1 x 500 ml, and 10 x 500 ml;
- Biofine bag: 1 x 100 ml, 10 x 100 ml, 20 x 100 ml, 1 x 250 ml, 10 x 250 ml, 1 x 500 ml, 12 x 500 ml, 1 x 1000 ml, and 6 x 1000 ml.
Not all packaging sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Fresenius Kabi AB SE-75174 Uppsala Sweden
Manufacturer
Fresenius Kabi AB Rapsgatan 7 SE-75174 Uppsala Sweden Fresenius Kabi Austria GmbH Hafnerstrasse 36 A-8055 Graz Austria For more detailed information, please contact the representative of the marketing authorization holder: Fresenius Kabi Polska Sp. z o.o. Al. Jerozolimskie 134 02-305 Warsaw tel.: +48 22 345 67 89
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Finland, France, Germany, Iceland, Ireland, Italy, Netherlands, Norway, Slovenia, Sweden, United Kingdom: SMOFlipid 200 mg/ml Cyprus, Czech Republic, Estonia, Greece, Hungary, Latvia, Lithuania, Luxembourg, Spain: SMOFlipid 20% Denmark, Poland, Portugal, Slovakia: SMOFlipid Date of last revision of the leaflet:06.03.2020
-----------------------------------------------------------------------------------------------------------------------------------
Information intended exclusively for healthcare professionals:
Warnings and precautions
During infusion, the triglyceride concentration in serum should not exceed 3 mmol/l.
Overdose may cause fat overload syndrome. Particular caution is advised in patients with increased risk of hyperlipidemia (e.g. patients receiving large amounts of fat, patients with severe sepsis, and premature infants with very low birth weight).
Administering only medium-chain fatty acids may cause metabolic acidosis. Concurrent administration of long-chain fatty acids contained in SMOFlipid significantly reduces this risk. Concurrent administration of carbohydrates may further reduce this risk. Therefore, it is recommended to administer carbohydrate solutions or amino acid solutions with carbohydrates concurrently. Regular laboratory tests related to monitoring of parenteral nutrition should be performed. This includes measuring blood glucose levels, liver function tests, acid-base balance, fluid balance, full blood count, and electrolyte levels.
This medicine contains soybean oil, fish oil, and egg phospholipids, which can rarely cause allergic reactions. Cross-allergic reactions have been observed between soy and peanuts.
In case of any signs of anaphylactic reaction (such as fever, chills, rash, or shortness of breath), the infusion should be stopped immediately.
SMOFlipid should be administered with caution in newborns and premature infants with hyperbilirubinemia and in cases of pulmonary hypertension. In newborns, especially premature infants, in cases of long-term parenteral nutrition, platelet count, liver enzyme activity, and triglyceride levels in serum should be monitored.
SMOFlipid contains up to 5 mmol of sodium per 1000 ml. This should be taken into account in patients controlling their sodium intake.
No other medicines or additional substances should be added to SMOFlipid, unless their compatibility has been established.
Method of administration
Infusion into a central or peripheral vein.
When used in newborns and children under 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration.
Instructions for use and handling Special warnings and precautions for use
Use only if the emulsion is homogeneous.
Exposure of parenteral nutrition solutions to light, especially after adding trace elements and (or) vitamins, may have undesirable effects on the clinical response in newborns, due to the formation of peroxides and other breakdown products. When used in newborns and children under 2 years of age, SMOFlipid should be protected from light until the end of administration.
Regarding the Biofine bag: before removing the outer protective bag, check the packaging integrity indicator (Oxalert). If the indicator is black, oxygen has penetrated the outer bag and the medicine should be discarded.
Before administration, check that the emulsion is homogeneous. Ensure that the emulsion ready for infusion does not show any signs of phase separation.
For single use only. Dispose of any unused residue.
After mixing with additional substances, SMOFlipid can be mixed aseptically with amino acid solutions, glucose solutions, and electrolyte solutions to obtain a total parenteral nutrition mixture of the "all-in-one" type.
On request, information on the compatibility of the medicine with additional substances and on the storage periods of prepared mixtures can be obtained from the marketing authorization holder. Any additional substances should be combined with the medicine under aseptic conditions. Any unused medicine or waste should be disposed of in accordance with local regulations.
Do not store at a temperature above 25°C. Do not freeze.
Storage after mixing If SMOFlipid is mixed with additional substances, the resulting mixture should be used immediately. Otherwise, the user is responsible for the storage period during use and the storage conditions before use. This period should not exceed 24 hours at a temperature of 2-8°C, unless the addition was made under controlled and validated aseptic conditions.
Special precautions for disposal and preparation of the medicinal product for use
When used in newborns and children under 2 years of age, protect from light until the end of administration. Exposure of SMOFlipid to light, especially after adding trace elements and (or) vitamins, leads to the formation of peroxides and other breakdown products, which can be limited by providing protection from light.
SMOFlipid - Instructions for preparing the Biofine bag for use
- 1.
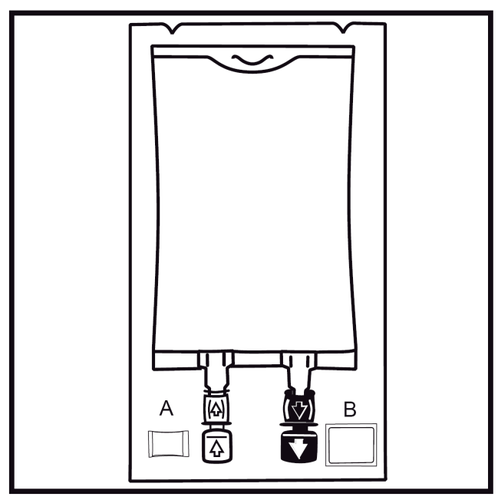
Check the packaging integrity indicator (Oxalert) Abefore removing the outer bag.
If the indicator is black, it means that the outer bag is damaged and the medicine should be discarded.
- 2.
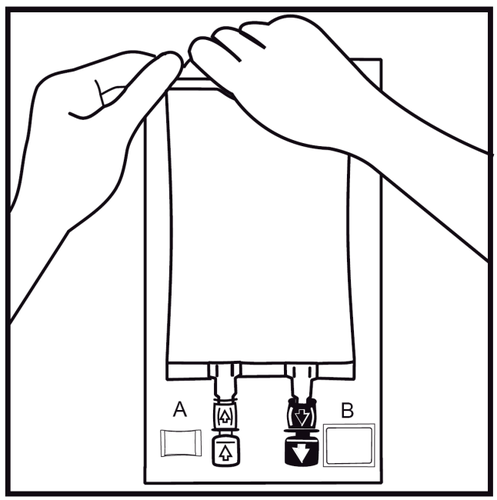
Remove the outer bag by tearing the seal at the top and pulling it along the packaging.
The packaging integrity indicator (Oxalert) Aand the oxygen absorber Bshould be removed.
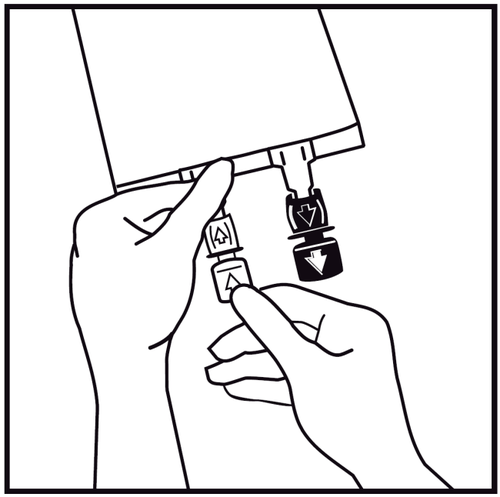
- 3.
In case of introducing additional substances, remove the one-time use plug marked with an arrow from the white port for adding these substances.
If no additional substances are introduced, proceed to step 5.
- 4.
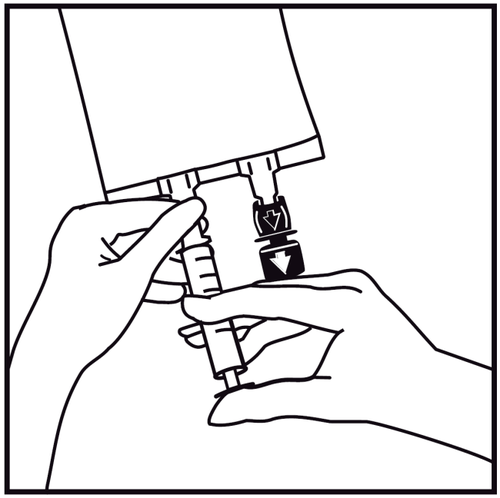
Insert the syringe needle horizontally through the center of the injection site and inject the additional substances (with established compatibility). Use syringes with needles with a diameter of 18 to 23 G and a maximum length of 40 mm.
- 5.
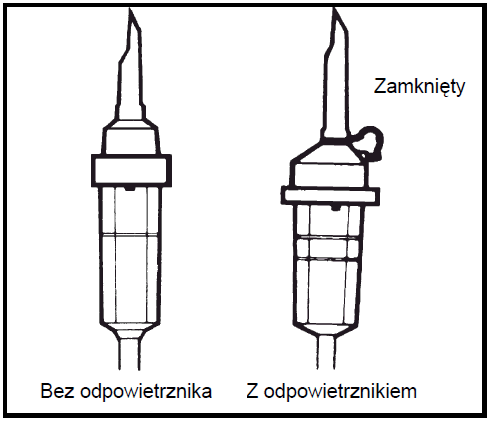
Use an infusion set without an air vent or close the air inlet in case of an infusion set with an air vent. Follow the instructions for use of the infusion set. Use an infusion set with a diameter specified in the ISO 8536-4 standard: 5.6 ± 0.1 mm.
- 6.
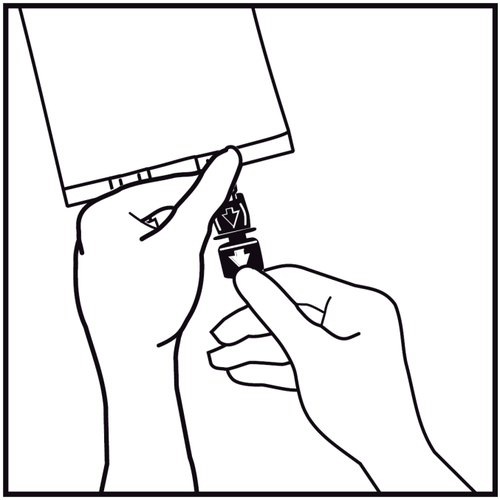
Remove the one-time use plug from the blue infusion port.
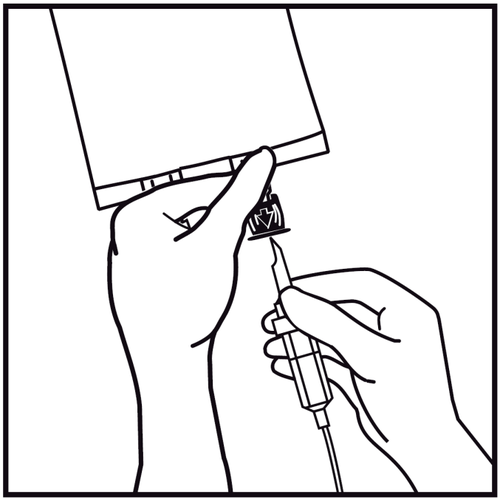
- 7.
Hold the base of the infusion port. Insert the tip of the infusion set into the infusion port and gently screw it in until it is fully inserted.
- 8.
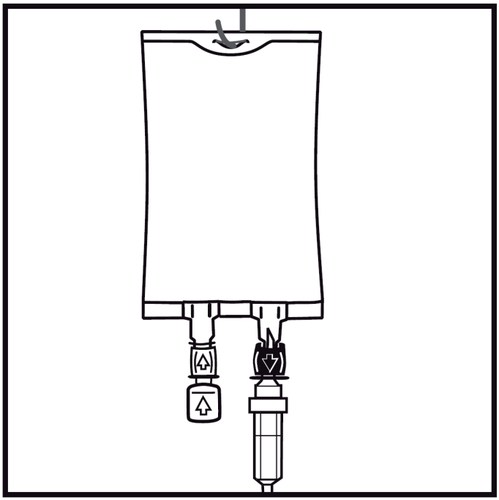
Hang the bag using the prepared opening and start the infusion.
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterFresenius Kabi AB Fresenius Kabi Austria GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SmoflipidDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not required
Alternatives to Smoflipid in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Smoflipid in Spain
Online doctors for Smoflipid
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Smoflipid – subject to medical assessment and local rules.














