
Sigletic

Ask a doctor about a prescription for Sigletic

How to use Sigletic
Leaflet accompanying the packaging: patient information
Sigletic, 25 mg, film-coated tablets
Sigletic, 50 mg, film-coated tablets
Sigletic, 100 mg, film-coated tablets
Sitagliptin
You should carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed to you by a doctor and should not be given to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Sigletic and what is it used for
- 2. Important information before taking Sigletic
- 3. How to take Sigletic
- 4. Possible side effects
- 5. How to store Sigletic
- 6. Contents of the packaging and other information
1. What is Sigletic and what is it used for
Sigletic contains the active substance sitagliptin, which belongs to a class of medicines called DPP-4 inhibitors (dipeptidyl peptidase-4 inhibitors), which reduce blood sugar levels in adults with type 2 diabetes.
This medicine helps to increase the amount of insulin released after a meal and reduce the amount of sugar produced by the body.
Your doctor has prescribed this medicine to reduce high blood sugar levels caused by type 2 diabetes. The medicine can be used alone or in combination with other medicines (insulin, metformin, sulfonylurea derivatives, or glitazones) that lower blood sugar levels, which may already be taken for diabetes, along with diet and exercise programs.
What is type 2 diabetes?
Type 2 diabetes is a disease in which the body does not produce enough insulin, and the insulin produced does not work as it should. The body may also produce too much sugar.
If this happens, sugar (glucose) builds up in the blood. This can lead to serious health problems, such as heart disease, kidney disease, vision loss, and limb amputation.
2. Important information before taking Sigletic
When not to take Sigletic
- if you are allergic to sitagliptin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
In patients taking Sigletic, cases of pancreatitis (see section 4) have been reported.
If you experience blisters on the skin, it may be a sign of a disease called bullous pemphigoid. Your doctor may advise you to stop taking Sigletic.
You should inform your doctor if you have or have had:
- pancreatic disease (e.g., pancreatitis);
- gallstones, alcohol dependence, or very high triglyceride levels in the blood. In such cases, the risk of pancreatitis (see section 4) may increase;
- type 1 diabetes;
- diabetic ketoacidosis (a complication of diabetes characterized by high blood sugar levels, rapid weight loss, nausea, or vomiting);
- any kidney disease that has occurred in the past or is currently present;
- an allergic reaction to Sigletic (see section 4).
Since this medicine does not work when blood sugar levels are low, it is unlikely to cause excessive lowering of blood sugar levels. However, if this medicine is taken with a sulfonylurea derivative or insulin, it may cause low blood sugar levels (hypoglycemia). Your doctor may reduce the dose of the sulfonylurea derivative or insulin.
Children and adolescents
Children and adolescents under 18 years of age should not take this medicine.
This medicine is not effective in children and adolescents aged 10 to 17 years. It is not known whether this medicine is safe and effective in children under 10 years of age.
Sigletic and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
In particular, you should inform your doctor if you are taking digoxin (a medicine used to treat heart rhythm disorders and other heart diseases). When taking Sigletic with digoxin, you should have your digoxin levels monitored.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before taking this medicine.
You should not take this medicine during pregnancy.
It is not known whether this medicine passes into breast milk. You should not take this medicine during breastfeeding or if you plan to breastfeed.
Driving and using machines
This medicine has no or negligible influence on the ability to drive and use machines. However, when driving or using machines, you should take into account that dizziness and drowsiness have been reported.
Taking this medicine with sulfonylurea derivatives or insulin may cause hypoglycemia, which can affect your ability to drive or use machines or work without safe foot support.
Sigletic contains sodium
Sigletic contains less than 1 mmol of sodium (23 mg) per dose, which means that the medicine is considered "sodium-free".
3. How to take Sigletic
This medicine should always be taken as directed by your doctor. If you are unsure, you should consult your doctor or pharmacist.
The recommended dose is:
- one 100 mg film-coated tablet;
- once a day;
- taken orally.
If you have kidney problems, your doctor may prescribe a lower dose of Sigletic (e.g., 25 mg or 50 mg).
This medicine can be taken with or without food and drinks.
Your doctor may prescribe this medicine alone or with other medicines that lower blood sugar levels.
Diet and exercise help your body use the sugar in your blood better. When taking Sigletic, it is essential to follow the diet and exercise program recommended by your doctor.
The 50 mg and 100 mg tablets of Sigletic can be divided into two equal doses.
If your doctor has prescribed half a 50 mg or 100 mg tablet, you should follow the instructions below for dividing the tablets.
- 1. Place the tablet on a flat, hard surface (e.g., a table, countertop), with the scored side facing up.
- 2. Break the tablet along the vertical score line, pressing it against the surface with your index fingers, as shown in figures 1 and 2.
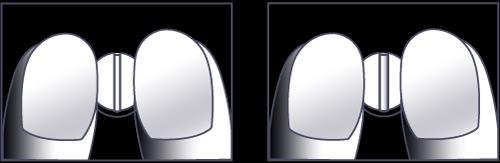
Figures 1 and 2: Dividing the Sigletic tablet into two equal doses.
Taking a higher dose of Sigletic than recommended
If you take more of this medicine than you should, you should contact your doctor immediately.
Missing a dose of Sigletic
If you miss a dose, you should take it as soon as possible. If it is almost time for your next dose, you should skip the missed dose and continue taking the medicine as usual. Do not take a double dose.
Stopping treatment with Sigletic
To maintain control of your blood sugar levels, you should take this medicine for as long as your doctor recommends. You should not stop taking this medicine without consulting your doctor first.
If you have any further questions about taking this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You should STOP taking Sigletic and contact your doctor immediately if you experience any of the following serious side effects:
- Severe and persistent abdominal pain (in the stomach area), which may radiate to the back, with or without nausea and vomiting - these may be symptoms of pancreatitis.
If you experience a severe allergic reaction (frequency not known), including rash, hives, blisters on the skin, or peeling of the skin, and swelling of the face, lips, tongue, and throat, which may cause difficulty breathing or swallowing, you should stop taking the medicine and contact your doctor immediately. Your doctor may prescribe a medicine to treat the allergic reaction and another medicine to treat your diabetes.
In some patients who added sitagliptin to metformin, the following side effects occurred:
Common (may affect up to 1 in 10 people): low blood sugar levels, nausea, bloating, vomiting.
Uncommon (may affect up to 1 in 100 people): stomach pain, diarrhea, constipation, drowsiness.
Some patients experienced various gastrointestinal symptoms after starting treatment with sitagliptin in combination with metformin (common).
In some patients who took sitagliptin in combination with a sulfonylurea derivative and metformin, the following side effects occurred:
Very common (may affect more than 1 in 10 people): low blood sugar levels.
Common: constipation.
In some patients who took sitagliptin and pioglitazone, the following side effects occurred:
Common: bloating, swelling of the hands or feet.
In some patients who took sitagliptin, pioglitazone, and metformin, the following side effects occurred:
Common: swelling of the hands or feet.
In some patients who took sitagliptin and insulin (with or without metformin), the following side effects occurred:
Common: flu.
Uncommon: dry mouth.
In some patients who took sitagliptin alone or with other anti-diabetic medicines in clinical trials or after the medicine was approved, the following side effects occurred:
Common: low blood sugar levels, headache, upper respiratory tract infections, stuffy or runny nose and sore throat, bone and joint pain, arm or leg pain.
Uncommon: dizziness, constipation, itching.
Rare: decreased platelet count.
Frequency not known: kidney disease (sometimes requiring dialysis), vomiting, joint pain, muscle pain, back pain, interstitial lung disease, bullous pemphigoid (a type of blisters on the skin).
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Sigletic
There are no special precautions for storing the medicine.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the blister and carton after: "EXP". The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation "EXP" indicates the expiry date, and after the abbreviation "Lot/LOT" indicates the batch number.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Sigletic contains
- The active substance of the medicine is sitagliptin. Each film-coated tablet contains sitagliptin phosphate monohydrate, equivalent to 25 mg, 50 mg, or 100 mg of sitagliptin.
- The other ingredients are: Tablet core: microcrystalline cellulose, calcium hydrogen phosphate, sodium carboxymethyl cellulose (type A), colloidal anhydrous silica, sodium stearyl fumarate. Tablet coating: polyvinyl alcohol, macrogol 3350, talc, titanium dioxide (E 171), yellow iron oxide (E 172), red iron oxide (E 172).
What Sigletic looks like and contents of the pack
Sigletic, 25 mg: light pink, round, biconvex film-coated tablet; tablet diameter 5.9 - 6.3 mm.
Sigletic, 50 mg: light orange, round, biconvex film-coated tablet with a score line on one side and "50" embossed on the other side; tablet diameter: 7.9 - 8.3 mm. The tablet can be divided into two equal doses.
Sigletic, 100 mg: light brown, round, biconvex film-coated tablet with a score line on one side and "100" embossed on the other side; tablet diameter 9.9-10.4 mm. The tablet can be divided into two equal doses.
PVC/PVDC/Aluminum blisters, in a cardboard box. Packs of 14, 28, 30, 56, 84, 90, 98 film-coated tablets.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
Pelplińska 19, 83-200 Starogard Gdański
phone +48 22 364 61 01
Manufacturer
Zakłady Farmaceutyczne Polpharma S.A.
Production Plant in Nowa Dęba
Metalowca 2
39-460 Nowa Dęba
Zakłady Farmaceutyczne POLPHARMA S.A.
Pelplińska 19, 83-200 Starogard Gdański
Date of last revision of the leaflet:November 2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A. Zakłady Farmaceutyczne POLPHARMA SA
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SigleticDosage form: Tablets, 25 mgActive substance: sitagliptinManufacturer: Lek Pharmaceuticals d.d. LEK S.A.Prescription requiredDosage form: Tablets, 50 mgActive substance: sitagliptinManufacturer: Lek Pharmaceuticals d.d. LEK S.A.Prescription requiredDosage form: Tablets, 100 mgActive substance: sitagliptinManufacturer: Lek Pharmaceuticals d.d. LEK S.A.Prescription required
Alternatives to Sigletic in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sigletic in Spain
Alternative to Sigletic in Ukraine
Online doctors for Sigletic
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sigletic – subject to medical assessment and local rules.










