
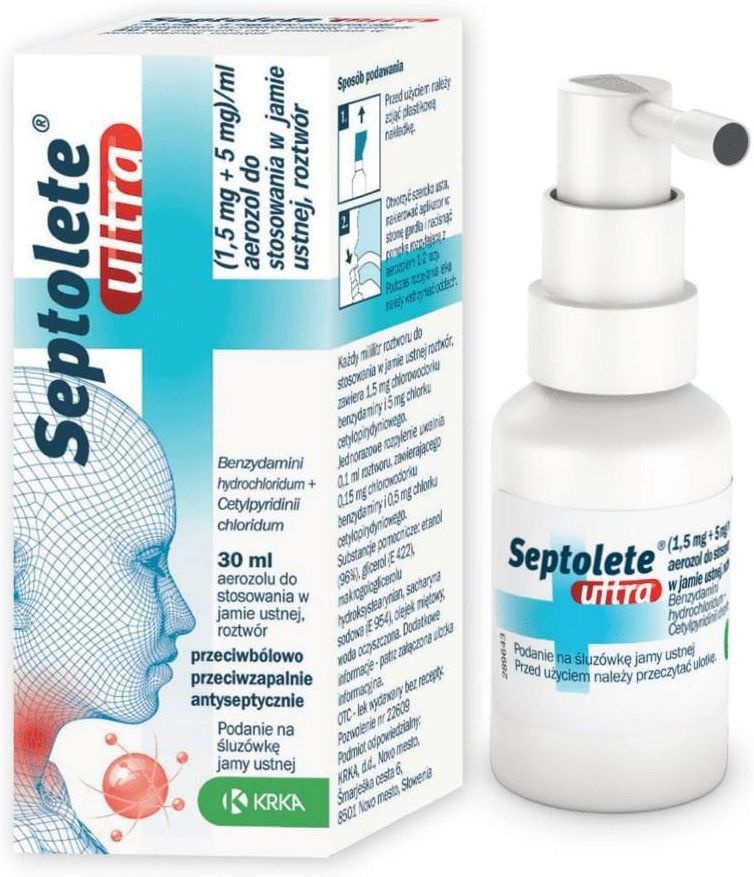
Septolete ultra

Ask a doctor about a prescription for Septolete ultra

How to use Septolete ultra
Package Leaflet: Information for the User
Septolete ultra, (1.5 mg + 5 mg)/ml, oral spray, solution
Benzydamine hydrochloride + Cetylpyridinium chloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this package leaflet for the patient or as directed by a doctor or pharmacist.
- Keep this package leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including those not listed in this package leaflet, tell your doctor or pharmacist. See section 4.
- If after 3 days there is no improvement or you feel worse, you should contact a doctor.
Package Leaflet Contents
- 1. What is Septolete ultra and what is it used for
- 2. Important information before using Septolete ultra
- 3. How to use Septolete ultra
- 4. Possible side effects
- 5. How to store Septolete ultra
- 6. Contents of the package and other information
1. What is Septolete ultra and what is it used for
Septolete ultra oral spray, solution is an anti-inflammatory, analgesic, and antiseptic for local use in the mouth. The oral spray, solution disinfects the mouth and throat and reduces the symptoms of throat inflammation, such as pain, redness, and swelling.
Septolete ultra oral spray, solution is used as an anti-inflammatory, analgesic, and antiseptic in the treatment of:
- irritation of the throat, mouth, and gums,
- in gum and throat inflammation
- and before and after tooth extraction.
If after 3 days there is no improvement or you feel worse, you should contact a doctor.
2. Important information before using Septolete ultra
When not to use Septolete ultra
- if you are allergic (hypersensitive) to benzydamine hydrochloride, cetylpyridinium chloride, or any of the other ingredients of this medicine (listed in section 6);
- in children under 6 years of age, as this pharmaceutical form is not intended for this age group.
Warnings and precautions
Before starting to use Septolete ultra, discuss it with your doctor or pharmacist. Septolete ultra should not be used for more than 7 days. If symptoms worsen or do not improve after 3 days, or if other symptoms such as fever appear, you should contact a doctor.
The use of local medicines, especially for a longer period, may lead to irritation. In such a case, treatment should be discontinued. Septolete ultra should not be used in combination with anionic compounds, such as those found in toothpastes, and it is not recommended to use the medicine directly before or after brushing teeth.
Avoid direct contact between Septolete ultra and the eyes. The medicine should not be inhaled.
Children and adolescents
Septolete ultra should not be used in children under 6 years of age, as this pharmaceutical form is not intended for this age group.
Septolete ultra and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take. Septolete ultra should not be used at the same time as other antiseptic products.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. It is not recommended to use Septolete ultra during pregnancy.
Before using Septolete ultra during breastfeeding, discuss it with your doctor. Your doctor will decide whether to stop breastfeeding or discontinue treatment.
Driving and using machines
Septolete ultra has no or negligible influence on the ability to drive and use machines.
Septolete ultra contains ethanol, hydroxyethylcellulose, and sodium
This medicine contains 267.60 mg of ethanol (alcohol) in 1 ml of oral spray, solution. The amount of alcohol in 1 ml of this medicine is equivalent to less than 7 ml of beer or 3 ml of wine. The small amount of alcohol in this medicine will not cause noticeable effects.
Hydroxyethylcellulose may cause stomach upset and diarrhea. This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means it is essentially 'sodium-free'.
3. How to use Septolete ultra
This medicine should always be used exactly as described in this package leaflet for the patient or as directed by a doctor or pharmacist. If you are unsure, consult your doctor or pharmacist.
Adults
To obtain a single dose, press the spray pump with the aerosol one totwo times. The dose can be repeated every 2 hours, 3-5 times a day.
Use in children and adolescents
Adolescents over 12 years of age
To obtain a single dose, press the spray pump with the aerosol one totwo times. The dose can be repeated every 2 hours, 3-5 times a day.
Children from 6 to 12 years of age
To obtain a single dose, press the spray pump with the aerosol one time. The dose can be repeated every 2 hours, 3-5 times a day.
Do not use Septolete ultra in children under 6 years of age.
Do not exceed the recommended dose
For optimal effect, it is not recommended to use the medicine directly before or after brushing teeth.
Method of administration
Before the first use of Septolete ultra, press the spray pump with the aerosol several times until a uniform mist is obtained. If the medicine has not been used for a longer period (at least one week), press the spray pump with the aerosol one time to obtain a uniform mist.
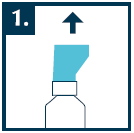
Before use, remove the plastic cap.
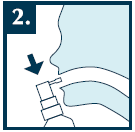
Open your mouth wide, direct the applicator towards the throat, and press the spray pump with the aerosol 1-2 times. While spraying the medicine, hold your breath.
A single press of the spray pump releases 0.1 ml of solution, containing 0.15 mg of benzydamine hydrochloride and 0.5 mg of cetylpyridinium chloride.
Duration of treatment
Do not use the medicine for more than 7 days. If symptoms worsen or do not improve after 3 days, or if other symptoms such as fever appear, you should consult a doctor.
Using more than the recommended dose of Septolete ultra
In case of using a higher dose than recommended or accidental ingestion of a large amount of medicine, you should immediately contact a doctor or pharmacist.
Missing a dose of Septolete ultra
Do not take a double dose to make up for a missed dose. If you have any further questions about using the medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Rare (may affect up to 1 in 1000 people)
- hives, increased sensitivity of the skin to sunlight (photosensitivity),
- sudden, uncontrolled narrowing of the airways in the lungs (bronchospasm).
Very rare (may affect up to 1 in 10,000 people)
- local irritation of the mouth, burning sensation in the mouth.
Frequency not known (cannot be estimated from the available data)
- allergic reaction (hypersensitivity): severe allergic reaction (anaphylactic shock), which may include difficulty breathing, pain or tightness in the chest and/or feeling of dizziness or fainting, severe itching of the skin or hives, swelling of the face, lips, tongue, and/or throat, and which may be life-threatening
- burning sensation of the oral mucosa, loss of sensation (numbness) of the oral mucosa.
Usually, these symptoms are temporary. However, if they occur, it is recommended to consult a doctor or pharmacist. By following the instructions in the package leaflet, you can reduce the risk of side effects.
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Septolete ultra
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date refers to the last day of the month stated. Do not store above 25°C. Shelf life after first opening of the immediate packaging: 1 year, provided the medicine has been stored below 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the package and other information
What Septolete ultra contains
- The active substances are benzydamine hydrochloride and cetylpyridinium chloride. Each ml of oral spray, solution contains 1.5 mg of benzydamine hydrochloride and 5 mg of cetylpyridinium chloride. One spray releases 0.1 ml of solution, containing 0.15 mg of benzydamine hydrochloride and 0.5 mg of cetylpyridinium chloride.
- The other ingredients are ethanol (96%), glycerol (E 422), sodium saccharin (E 954), hydroxyethylcellulose, peppermint oil, and purified water. See section 2 "Septolete ultra contains ethanol, hydroxyethylcellulose, and sodium".
What Septolete ultra looks like and contents of the pack
Septolete ultra is a clear, colorless to yellowish solution. Septolete ultra is available in a cardboard box containing 30 ml of oral spray, solution in a bottle with a spray pump and applicator, and a cap. 30 ml of oral spray, solution is sufficient for 250 sprays.
Marketing authorization holder and manufacturer
KRKA, d.d., Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Slovenia
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
For further information about this medicine, please contact the local representative of the marketing authorization holder:
KRKA-POLSKA Sp. z o.o., ul. Równoległa 5, 02-235 Warsaw
Date of last revision of the package leaflet:
| Czech Republic | Septabene 1,5 mg /ml + 5,0 mg /ml oral spray, solution |
| Bulgaria | Septolete total 1.5 mg/ml + 5.0 mg/ml oromucosal spray |
| Estonia | Septolete omni |
| Hungary | Septolete extra 1,5 mg/ 5,0 mg/1 ml oral spray, solution |
| Croatia | Septolete duo 1,5 mg/ml + 5,0 mg/ml spray for oral mucosa |
| Ireland | Septabene 1.5 mg/ml + 5.0 mg/ml oromucosal spray, solution |
| Italy | Septafar |
| Lithuania | Septabene 1,5 mg/5,0 mg/ml oral spray, solution |
| Latvia | Septabene 1,5 mg/5,0 mg/ml oral spray, solution |
| Portugal | Septolete duo 1.5 mg/ml + 5.0 mg/ml solution for oral spray |
| Romania | Septolete omni 1.5 mg/ml + 5.0 mg/ml oromucosal spray, solution |
| Slovenia | Septabene 1.5 mg/ml + 5.0 mg/ml oral spray, solution |
| Slovakia | Septolete extra 1,5 mg/5mg/1 ml |
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterKrka, d.d., Novo mesto
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Septolete ultraDosage form: Aerosol, 1.5 mg/mlActive substance: benzydaminePrescription not requiredDosage form: Aerosol, 3 mg/mlActive substance: benzydamineManufacturer: Laboratorios Bohm SA Laboratorium Sanitatis S.L. MCM Klosterfrau Vertriebsgesellschaft mbHPrescription not requiredDosage form: Lozenge, 3 mgActive substance: benzydaminePrescription not required
Alternatives to Septolete ultra in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Septolete ultra in Spain
Alternative to Septolete ultra in Ukraine
Online doctors for Septolete ultra
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Septolete ultra – subject to medical assessment and local rules.







