
Risperidone Teva

Ask a doctor about a prescription for Risperidone Teva

How to use Risperidone Teva
Leaflet accompanying the packaging: information for the user
Risperidone Teva, 25 mg, powder and solvent for prolonged-release injection suspension
Risperidone Teva, 37.5 mg, powder and solvent for prolonged-release injection suspension
Risperidone Teva, 50 mg, powder and solvent for prolonged-release injection suspension
Risperidone
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Risperidone Teva and what is it used for
- 2. Important information before using Risperidone Teva
- 3. How to use Risperidone Teva
- 4. Possible side effects
- 5. How to store Risperidone Teva
- 6. Package contents and other information
1. What is Risperidone Teva and what is it used for
Risperidone Teva belongs to a group of antipsychotic medicines.
Risperidone Teva is used to treat schizophrenia, a condition where the patient sees, hears, or feels things that do not exist, believes in untrue things, feels unusually suspicious or confused.
Risperidone Teva is intended for patients currently being treated with oral antipsychotic medications (e.g., tablets, capsules).
Risperidone Teva may help alleviate symptoms of the disease and prevent their recurrence.
2. Important information before using Risperidone Teva
When not to use Risperidone Teva
- if the patient is allergic to risperidone or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
- if the patient has never used risperidone before in any form, they should first use risperidone orally before starting treatment with Risperidone Teva.
Before starting treatment with Risperidone Teva, the patient should discuss the following with their doctor or pharmacist:
- if the patient has heart rhythm disorders, such as an irregular heartbeat, or if they have a tendency to low blood pressure, or are taking blood pressure medications. Risperidone Teva may cause a decrease in blood pressure. It may be necessary to adjust the dose of the medicine
- if the patient knows of any factors that may contribute to their risk of stroke, such as high blood pressure, cardiovascular disease, or cerebral vascular disorders
- if the patient has ever experienced involuntary movements of the tongue, lips, or face
- if the patient has ever experienced a condition characterized by fever, severe muscle stiffness, sweating, or decreased level of consciousness (also known as malignant neuroleptic syndrome)
- if the patient has Parkinson's disease or dementia
- if the patient has had a low white blood cell count in the past (which may or may not have been caused by the use of other medications)
- if the patient has diabetes
- if the patient has epilepsy
- if the patient is a man and has experienced a prolonged or painful erection
- if the patient has disorders of body temperature regulation or overheats
- if the patient has kidney or liver function disorders
- if the patient has an abnormally high level of the hormone prolactin in their blood or a suspected prolactin-dependent tumor
- if the patient or any of their relatives have had blood clots in the past, as the use of such medications as Risperidone Teva is associated with the formation of blood clots.
If the patient is unsure whether any of the above conditions apply to them, they should consult their doctor or pharmacist before using Risperidone Teva.
The attending physician may order a white blood cell count test, as very rare cases of a dangerously low number of a certain type of white blood cell necessary for fighting infections have been observed in patients using Risperidone Teva.
Even if the patient has previously tolerated oral risperidone, rare allergic reactions may occur after injections of Risperidone Teva. The patient should seek medical help immediately if they experience: rash, throat swelling, itching, or difficulty breathing, as these may be symptoms of a severe allergic reaction.
Risperidone Teva may cause weight gain. Significant weight gain can have a negative impact on health. The attending physician will regularly monitor the patient's weight.
The doctor should check if the patient has symptoms of high blood sugar levels, as patients using risperidone have been observed to develop diabetes and exacerbate existing diabetes. In patients with existing diabetes, blood sugar levels should be regularly monitored.
Risperidone Teva often increases the level of the hormone prolactin in the blood. This can cause side effects such as: menstrual disorders, fertility problems in women, breast swelling in men (see section "Possible side effects"). If such side effects occur, it is recommended to perform a blood prolactin level test.
During cataract surgery, the pupil (the black circle in the center of the eye) may not dilate sufficiently. The iris (the colored part of the eye) may also be floppy during the procedure, which can result in eye damage. If the patient is scheduled for eye surgery, they should inform their ophthalmologist about their use of this medicine.
Elderly patients with dementia
Risperidone Teva should not be given to elderly patients with dementia.
Medical help should be sought immediately if the patient or their caregiver notices a sudden change in the patient's mental state, sudden muscle weakness or numbness of the face, arms, or legs, especially if it is one-sided or speech disorders, even if they occur for a short time. These symptoms may signal a stroke.
Patients with kidney or liver function disorders
Studies have been conducted on the oral use of risperidone in patients with kidney or liver function disorders, but no studies have been conducted on the use of Risperidone Teva. In this group of patients, Risperidone Teva should be used with caution.
Risperidone Teva and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Patients should especially inform their doctor or pharmacist if they are taking any of the following medicines:
- sedatives acting on the brain (benzodiazepines) or certain painkillers (opioids), antihistamines, as risperidone may enhance their sedative effect
- medicines that may cause changes in heart rhythm, such as malaria medications, anti-arrhythmic medications, antihistamines, or certain antidepressants
- medicines that slow down the heart rate
- medicines that lower potassium levels in the blood (e.g., certain diuretics)
- medicines used to treat Parkinson's disease (e.g., levodopa)
- medicines that increase the activity of the central nervous system (psychostimulants, such as methylphenidate)
- medicines used to treat high blood pressure. Risperidone Teva may lower blood pressure
- diuretics (used in heart disease or to reduce swelling in areas where there is excessive fluid accumulation, e.g., furosemide or chlorothiazide). Risperidone Teva used alone or in combination with furosemide may increase the risk of stroke or death in elderly patients with dementia.
The following medicines may weaken the effect of risperidone:
- rifampicin (a medicine used to treat certain infections)
- carbamazepine, phenytoin (anti-epileptic medicines)
- phenobarbital. When starting or stopping these medicines, it may be necessary to adjust the dose of risperidone.
The following medicines may enhance the effect of risperidone:
- quinidine (used in certain heart diseases)
- antidepressants, such as paroxetine, fluoxetine, tricyclic antidepressants
- beta-adrenergic blockers (used to treat high blood pressure)
- phenothiazines (e.g., used to treat psychoses or to sedate)
- cimetidine, ranitidine (reducing stomach acid)
- itraconazole and ketoconazole (used in fungal infections)
- certain medicines used to treat HIV/AIDS, such as ritonavir
- verapamil, used to treat high blood pressure and/or heart rhythm disorders
- sertraline and fluvoxamine, used to treat depression and other mental disorders. When starting or stopping these medicines, it may be necessary to adjust the dose of risperidone.
If the patient is unsure whether they have taken or are taking any of the above medicines, they should consult their doctor or pharmacist before using Risperidone Teva.
Risperidone Teva with food, drink, and alcohol
The patient should not drink alcohol while using Risperidone Teva.
Pregnancy, breastfeeding, and fertility
- in newborns whose mothers used Risperidone Teva in the last trimester (last 3 months of pregnancy), the following symptoms may occur: trembling, muscle stiffness, and/or weakness, drowsiness, agitation, breathing difficulties, and feeding difficulties. If such symptoms are observed in the child, the patient should contact their doctor.
- Risperidone Teva may increase the level of prolactin in the blood - a hormone that can affect fertility (see section "Possible side effects").
Driving and using machines
While using Risperidone Teva, the patient may experience dizziness, fatigue, and vision disturbances. Therefore, without consulting their doctor, the patient should not drive vehicles, use tools, or operate machines.
Risperidone Teva contains sodium
The medicine contains less than 1 mmol of sodium (23 mg), which means it is considered "sodium-free".
3. How to use Risperidone Teva
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
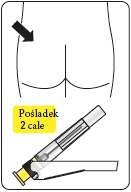
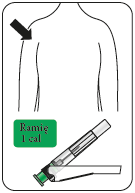
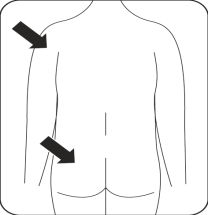
Risperidone Teva is administered by intramuscular injection into the arm or buttock every 2 weeks by a doctor or nurse. Injections should be given alternately, once on the right side and once on the left side. It should not be administered intravenously.
Recommended dose:
Initial dose
If the patient has been taking oral risperidone in the last 2 weeks at a daily dose of 4 mg or less, the initial dose of Risperidone Teva should be 25 mg.
If the patient has been taking oral risperidone in the last 2 weeks at a daily dose greater than 4 mg, the initial dose of Risperidone Teva may be 37.5 mg.
If the patient is currently being treated with an antipsychotic medication other than risperidone, the initial dose of Risperidone Teva will depend on their current therapy. The doctor will choose and prescribe Risperidone Teva at a dose of 25 mg or 37.5 mg.
The doctor will adjust the dose of Risperidone Teva according to the patient's needs.
Maintenance dose:
- Usually, a dose of 25 mg is administered by injection every 2 weeks.
- If necessary, a higher dose of 37.5 mg or 50 mg is administered. The doctor will adjust the dose of Risperidone Teva according to the patient's needs.
- The doctor may also recommend taking oral risperidone for 3 weeks after the first injection.
Using a higher dose of Risperidone Teva than recommended
- In patients who have used a higher dose of Risperidone Teva than they should have, the following symptoms have been observed: drowsiness, fatigue, abnormal body movements, difficulty maintaining balance and walking, dizziness due to low blood pressure, and abnormal heart rhythm. There have also been reports of abnormal heart conduction and seizures.
- The patient should consult their doctor immediately.
Stopping treatment with Risperidone Teva
The effect of the medicine will disappear if the patient stops using it. Therefore, without explicit instruction from the doctor, the patient should not stop using the medicine, as this may lead to a recurrence of the disease.
The patient should always report to the doctor's office every 2 weeks for their next injection.
If the patient is unable to attend an appointment, they should immediately notify their doctor to arrange an alternative appointment.
In case of any further doubts about the use of this medicine, the patient should consult their doctor or pharmacist.
Use in children and adolescents
Risperidone Teva is not intended for persons under 18 years of age.
4. Possible side effects
Like all medicines, Risperidone Teva can cause side effects, although not everybody gets them.
The patient should immediately inform their doctor if they experience any of the following:
uncommon side effects(may affect up to 1 in 100 people):
- in patients with dementia, a sudden change in mental state or sudden muscle weakness or numbness of the face, arms, or legs, especially if it is one-sided or speech disorders, even if they occur for a short time. These symptoms may signal a stroke
- late dyskinesia (involuntary, repetitive body movements). The patient should immediately inform their doctor if they experience involuntary, rhythmic movements of the tongue, lips, or face. It may be necessary to discontinue Risperidone Teva.
The patient should immediately inform their doctor if they experience any of the following:
rare side effects(may affect up to 1 in 1,000 people):
- blood clots in the veins, especially in the legs (symptoms include pain and redness of the leg); these clots can move through the blood vessels to the lungs, causing chest pain and difficulty breathing. If such symptoms occur, the patient should seek medical help immediately
- fever, muscle stiffness, sweating, or decreased level of consciousness (a condition known as malignant neuroleptic syndrome). Immediate treatment may be necessary
- a man experiences a prolonged or painful erection. This condition is known as priapism. Immediate treatment may be necessary
- a severe allergic reaction characterized by fever, swelling of the lips, face, or tongue, difficulty breathing, itching, rash, or low blood pressure. Even if the patient has previously tolerated oral risperidone, rare allergic reactions may occur after injections of Risperidone Teva.
Other side effects may also occur:
very common side effects(may affect more than 1 in 10 people):
- cold symptoms
- difficulty sleeping or waking up
- depression, anxiety
- parkinsonism: this condition may include slow or abnormal movements, a feeling of stiffness or muscle tension (which can cause the patient's movements to be uneven), and sometimes even a feeling of "freezing" of movements, followed by a release. Other symptoms of parkinsonism include: a slow, shuffling gait, tremors, increased saliva production, and a mask-like face.
- headache.
common side effects(may affect up to 1 in 10 people):
- pneumonia, respiratory tract infection (bronchitis), sinus infection
- urinary tract infection, flu-like symptoms, anemia
- increased prolactin levels in the blood (with or without symptoms). Symptoms of increased prolactin levels occur uncommonly and may include in men: breast swelling, difficulty achieving or maintaining an erection, decreased sex drive, or other sexual disorders. In women, they may include breast discomfort, milk secretion, absence of menstruation, or other menstrual disorders or fertility disorders.
- high blood sugar, weight gain, increased appetite, weight loss, decreased appetite
- sleep disturbances, irritability, decreased sex drive, anxiety, drowsiness, or decreased alertness
- dystonia: in this condition, there are slow or sustained involuntary muscle contractions. They can affect any part of the body (which can result in an abnormal posture), but dystonia most commonly affects the facial muscles, including abnormal eye movements, lip, tongue, or jaw movements.
- dizziness
- dyskinesia: in this condition, there are involuntary muscle movements, including repetitive, spasmodic, or twisting movements or jerks
- tremors
- blurred vision
- rapid heartbeat
- low blood pressure, chest pain, high blood pressure
- shortness of breath, sore throat, cough, stuffy nose
- abdominal pain, discomfort in the abdomen, vomiting, nausea, gastrointestinal infection, constipation, diarrhea, indigestion, dry mouth, toothache
- rash
- muscle spasms, bone or muscle pain, back pain, joint pain
- urinary incontinence
- erectile dysfunction
- absence of menstruation
- milk secretion
- body, upper, or lower limb swelling, fever, weakness, fatigue (exhaustion)
- pain
- reaction at the injection site, including itching, pain, or swelling
- increased liver enzyme activity in the blood, increased activity of the enzyme GGT (liver enzyme - gamma-glutamyltransferase) in the blood
- fall.
uncommon side effects(may affect up to 1 in 100 people):
- respiratory tract infection, urinary tract infection, ear infection, eye infection, tonsillitis, fungal infection of the nails, skin infection, localized skin infection, viral infection, scabies, subcutaneous abscess
- decreased white blood cell count, decreased platelet count (blood cells that help stop bleeding), decreased red blood cell count
- allergic reaction
- presence of sugar in the urine, development of diabetes, or worsening of existing diabetes
- loss of appetite leading to malnutrition and low body weight
- increased triglyceride levels (fats) in the blood, increased cholesterol levels in the blood
- irritable mood (mania), confusion, inability to achieve orgasm, nervousness, nightmares
- loss of consciousness, seizures, fainting
- involuntary movement of body parts, balance disorders, coordination disorders, dizziness when changing position to standing, concentration disorders, speech difficulties, loss or abnormal taste, decreased sensation of pain and touch on the skin, tingling, numbness, or prickling of the skin
- eye infection or conjunctivitis, dry eye, increased tearing, redness of the eyes
- vertigo, ringing in the ears, ear pain
- atrial fibrillation (irregular heartbeat), atrioventricular block (impaired conduction of impulses between the upper and lower parts of the heart), abnormal conduction of electrical impulses in the heart, prolonged QT interval in the heart, slow heart rate, abnormal electrocardiogram (ECG), feeling of pounding or fluttering in the chest (palpitations)
- low blood pressure when changing position to standing (which may cause some patients using Risperidone Teva to faint, feel dizzy, or lose consciousness)
- rapid, shallow breathing, congestion of the airways, wheezing, nosebleeds
- incontinence of feces, difficulty swallowing, excessive gas
- itching, hair loss, rash, dry skin, redness of the skin, skin discoloration, acne, flaky, itchy scalp or body
- increased creatine kinase activity in the blood, an enzyme that is sometimes released from damaged muscles
- joint stiffness, joint swelling, muscle weakness, neck pain
- frequent urination, inability to urinate, painful urination
- ejaculation disorders, delayed menstruation, absence of menstruation, and other menstrual disorders (in women), breast swelling in men, sexual disorders, breast pain, breast discomfort
- face, lip, eye, or tongue swelling
- chills, increased body temperature
- change in gait
- thirst, malaise, chest discomfort, feeling unwell
- skin hardening
- increased liver enzyme activity in the blood
- pain related to medical procedures.
rare side effects(may affect up to 1 in 1,000 people):
- decreased number of a certain type of white blood cell necessary for fighting infections
- abnormal secretion of the hormone regulating urine volume
- low blood sugar
- excessive water drinking
- sleepwalking (sleepwalking)
- eating disorders related to sleep
- lack of movement or reaction while awake (catatonia)
- lack of emotions
- decreased level of consciousness
- tremors, nodding movements of the head
- eye movement disorders, rotational eye movements, hypersensitivity to light
- complications during cataract surgery. During this procedure, a condition known as intraoperative floppy iris syndrome (IFIS) may occur if the patient is using or has used Risperidone Teva. If the patient is scheduled for cataract surgery, they should inform their ophthalmologist about their use of this medicine.
- irregular heartbeat
- dangerously low number of a certain type of white blood cell necessary for fighting infections, increased number of eosinophils (a type of white blood cell) in the blood
- sleep apnea (breathing disorders during sleep)
- aspiration pneumonia (caused by food entering the airways), pulmonary congestion, wheezing sounds from the lungs, voice disorders, respiratory disorders
- pancreatitis, intestinal obstruction
- very hard stools
- drug rash
- hives, skin thickening, dandruff, skin disorders, skin damage
- muscle fiber breakdown and muscle pain (rhabdomyolysis)
- abnormal posture
- breast enlargement, milk secretion
- low body temperature, discomfort
- jaundice (yellowing of the skin and eyes)
- dangerously excessive water drinking
- increased insulin levels in the blood (a hormone that regulates blood sugar levels)
- cerebrovascular disorders
- lack of response to stimuli
- diabetic coma due to uncontrolled diabetes
- sudden loss of vision or blindness
- glaucoma (increased pressure in the eyeball), eyelid margin ulcers (with crust formation)
- flushing, tongue swelling
- dry lips
- breast enlargement
- low body temperature, cooling of hands and feet
- withdrawal symptoms.
very rare side effects(may affect up to 1 in 10,000 people):
- life-threatening complications due to uncontrolled diabetes
- severe allergic reaction with swelling, which may involve the throat and lead to difficulty breathing
- intestinal obstruction, which can lead to intestinal blockage.
frequency not known(cannot be estimated from the available data):
- severe or life-threatening rash with blisters and peeling of the skin, which may start in the mouth, nose, eyes, or genitals and spread to other areas of the body (Stevens-Johnson syndrome or toxic epidermal necrolysis).
A side effect observed with the use of another medicine, paliperidone, which is very similar to risperidone and may also occur with the use of Risperidone Teva: rapid heartbeat when changing position to standing.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the patient should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Risperidone Teva
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
The entire package should be stored in the refrigerator (2°C - 8°C). Outside the refrigerator, Risperidone Teva can be stored at a temperature below 25°C for a maximum of 7 days before administration.
Store in the original packaging to protect from light.
After reconstitution:Chemical and physical stability for 24 hours at 25°C has been demonstrated.
From a microbiological point of view, the medicine should be administered immediately after preparation.
If it is not used immediately, the user is responsible for the storage time and conditions. The reconstituted suspension can be stored for no more than 6 hours at 25°C, unless the preparation of the suspension was carried out under controlled and validated aseptic conditions.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Risperidone Teva contains
The active substance of the medicine is risperidone.
Each vial of powder for prolonged-release injection suspension of Risperidone Teva contains 25 mg, 37.5 mg, or 50 mg of risperidone.
The other ingredients are:
Powder ingredients:
Poly-(d,l-lactide-co-glycolide)
Solvent ingredients:
Polysorbate 20
Sodium carmellose
Disodium phosphate dihydrate
Citric acid
Sodium chloride
Sodium hydroxide
Water for injections
What Risperidone Teva looks like and what the package contains
Risperidone Teva, 25 mg
Each single-dose package (kit) contains the following components placed together on a plastic tray:
- one small vial with a gray stopper, closed with a pink aluminum flip-off cap, containing the powder (which contains the active substance risperidone).
- one ampoule syringe with a clear, colorless liquid (2 ml) to be added to the powder to prepare the prolonged-release injection suspension;
- one vial adapter to prepare the suspension;
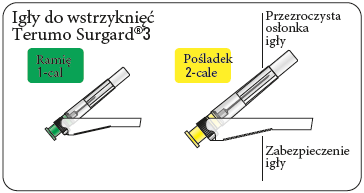
- two Terumo SurGuard®3 needles for intramuscular injection: one 21G UTW 1-inch (0.8 mm x 25 mm) needle with a safety device for injection into the deltoid muscle; one 20G TW 2-inch (0.9 mm x 51 mm) needle with a safety device for injection into the gluteal muscle.
Risperidone Teva, 37.5 mg
Each single-dose package (kit) contains the following components placed together on a plastic tray:
- one small vial with a gray stopper, closed with a green aluminum flip-off cap, containing the powder (which contains the active substance risperidone).
- one ampoule syringe with a clear, colorless liquid (2 ml) to be added to the powder to prepare the prolonged-release injection suspension;
- one vial adapter to prepare the suspension;
- two Terumo SurGuard®3 needles for intramuscular injection: one 21G UTW 1-inch (0.8 mm x 25 mm) needle with a safety device for injection into the deltoid muscle; one 20G TW 2-inch (0.9 mm x 51 mm) needle with a safety device for injection into the gluteal muscle.
Risperidone Teva, 50 mg
Each single-dose package (kit) contains the following components placed together on a plastic tray:
- one small vial with a gray stopper, closed with a blue aluminum flip-off cap, containing the powder (which contains the active substance risperidone).
- one ampoule syringe with a clear, colorless liquid (2 ml) to be added to the powder to prepare the prolonged-release injection suspension;
- one vial adapter to prepare the suspension;
- two Terumo SurGuard®3 needles for intramuscular injection: one 21G UTW 1-inch (0.8 mm x 25 mm) needle with a safety device for injection into the deltoid muscle; one 20G TW 2-inch (0.9 mm x 51 mm) needle with a safety device for injection into the gluteal muscle.
Risperidone Teva is available in a package containing 1, 2, or 5 kits.
Marketing authorization holder:
Teva B.V.
Swensweg 5
2031GA Haarlem
Netherlands
Manufacturer:
PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.)
Prilaz baruna Filipovića 25
10000 Zagreb
Croatia
Pharmathen International S.A
Industrial Park Sapes,
Rodopi Prefecture, Block No 5,
Rodopi 69300,
Greece
Pharmathen S.A
Dervenakion 6,
Pallini Attiki,
15351, Greece
To obtain more detailed information about this medicine, the patient should contact their local representative of the marketing authorization holder.
Teva Pharmaceuticals Polska Sp. z o.o., ul. Emilii Plater 53, 00-113 Warsaw, phone +48 22 345 93 00
This medicine is authorized in the Member States of the European Economic Area under the following names:
Member State
Marketing authorization holder
Austria
Risperidon ratiopharm 25 mg powder and solvent for prolonged-release injection suspension
Risperidon ratiopharm 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidon ratiopharm 50 mg powder and solvent for prolonged-release injection suspension
Belgium
Risperidone Teva 25 mg, 37.5 mg & 50 mg powder and solvent for injection suspension with prolonged release/ Poudre et solvant pour suspension injectable à libération prolongée/ Pulver und Lösungsmittel zur Herstellung einer Depot-Injektionssuspension
Bulgaria
Сперидан 37.5 mg powder and solvent for injection suspension with prolonged release
Сперидан 50 mg powder and solvent for injection suspension with prolonged release
Germany
Risperidon-ratiopharm 25 mg powder and solvent for prolonged-release injection suspension
Risperidon-ratiopharm 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidon-ratiopharm 50 mg powder and solvent for prolonged-release injection suspension
Denmark
Risperidone Teva GmbH
Estonia
RISPERIDONA TEVA 25 MG powder and solvent for prolonged-release injection suspension EFG
RISPERIDONA TEVA 37.5 MG powder and solvent for prolonged-release injection suspension EFG
RISPERIDONA TEVA 50 MG powder and solvent for prolonged-release injection suspension EFG
Finland
Risperidon ratiopharm 25 mg powder and solvent for prolonged-release injection suspension
Risperidon ratiopharm 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidon ratiopharm 50 mg powder and solvent for prolonged-release injection suspension
France
RISPERIDONE Teva L.P. 25 mg/2ml, powder and solvent for prolonged-release injection suspension in a pre-filled syringe
RISPERIDONE Teva L.P. 37.5 mg/2 ml, powder and solvent for prolonged-release injection suspension in a pre-filled syringe
RISPERIDONE Teva L.P. 50 mg/2 ml, powder and solvent for prolonged-release injection suspension in a pre-filled syringe
Croatia
Risset 25 mg powder and solvent for injection suspension with prolonged release
Risset 37.5 mg powder and solvent for injection suspension with prolonged release
Risset 50 mg powder and solvent for injection suspension with prolonged release
Hungary
Risperidone Teva 25 mg powder and solvent for prolonged-release injection suspension
Risperidone Teva 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidone Teva 50 mg powder and solvent for prolonged-release injection suspension
Iceland
Risperidone Teva GmbH
Italy
Risperidone Teva Group 25 mg powder and solvent for prolonged-release injection suspension
Risperidone Teva Group 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidone Teva Group 50 mg powder and solvent for prolonged-release injection suspension
Lithuania
Risperidone Teva 25 mg powder and solvent for prolonged-release injection suspension
Risperidone Teva 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidone Teva 50 mg powder and solvent for prolonged-release injection suspension
Luxembourg
Risperidon-ratiopharm 25 mg powder and solvent for prolonged-release injection suspension
Risperidon-ratiopharm 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidon-ratiopharm 50 mg powder and solvent for prolonged-release injection suspension
Netherlands
Risperidon Teva 25 mg, powder and solvent for injection suspension with prolonged release
Risperidon Teva 37.5 mg, powder and solvent for injection suspension with prolonged release
Risperidon Teva 50 mg, powder and solvent for injection suspension with prolonged release
Norway
Risperidone Teva GmbH
Poland
Risperidone Teva
Portugal
Risperidona ratiopharm
Romania
Risperidonă Teva 25 mg powder and solvent for injection suspension with prolonged release
Risperidonă Teva 37.5 mg powder and solvent for injection suspension with prolonged release
Risperidonă Teva 50 mg powder and solvent for injection suspension with prolonged release
Sweden
Risperidone Teva GmbH
Slovenia
Risset 25 mg powder and solvent for injection suspension with prolonged release
Risset 37.5 mg powder and solvent for injection suspension with prolonged release
Risset 50 mg powder and solvent for injection suspension with prolonged release
Slovakia
Risperidon Teva 25 mg powder and solvent for prolonged-release injection suspension
Risperidon Teva 37.5 mg powder and solvent for prolonged-release injection suspension
Risperidon Teva 50 mg powder and solvent for prolonged-release injection suspension
Date of last revision of the leaflet: March 2022
-----------------------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals only
Important information
To ensure the successful administration of Risperidone Teva, the "Instructions for use of the device" should be carefully read (step by step).
Use only the components of the kit
The components of this kit are specifically designed for use with Risperidone Teva. Only the solvent provided in the packaging should be used to prepare the Risperidone Teva suspension.
Do notreplace anyof the kit components in the packaging.
Do not store the suspension after preparation
The suspension should be administered as soon as possible after preparation to avoid sedimentation.
Proper administration
The entire contents of the vial must be administered to ensure the patient receives the correct dose of Risperidone Teva.

Do not reuse
In order for medical devices to function as intended, specific material properties are required. These properties have been checked only for single use. Any attempts to reuse the device may lead to its damage or deterioration of performance.
Package contents (kit)
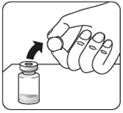
Step 1 Assemble the kit
Remove packaging
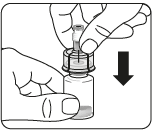
Connect the vial to the connector (adapter)
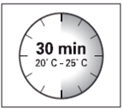
Wait
30 minutes
Before
preparing
the suspension, remove
the product packaging
from the refrigerator
and leave it at
room temperature for at
least 30 minutes.
Do not heat it in
any other way.
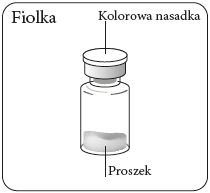
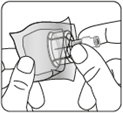
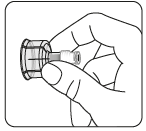
Remove the vial nozzle
Remove the colored
nozzle from the vial.
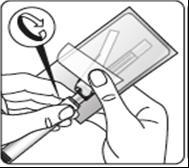
Wipe the gray rubber stopper
from the top with an
alcohol-soaked swab.
Let it dry.
Do notremove
the gray rubber stopper.
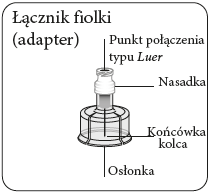
Prepare the vial connector (adapter)
Tear off the blister pack
and remove the vial connector,
holding it between the white nozzle
and the shield.
Nevertouch the needle tip
or the Luer connection point.
This will contaminate it.
Connect the adapter to the vial
Place the vial on a hard
surface and hold it at
the base. Position the adapter
centrally over the gray rubber stopper.
Press the adapter straight down until it clicks firmly onto the top of the vial, which will be confirmed by a audible "click".
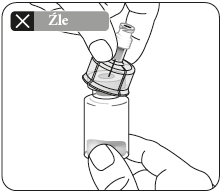
Do notput the adapter on the vial at an angle, as this may cause the solvent to leak during administration.
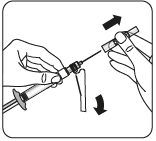
Connecting the syringe to the adapter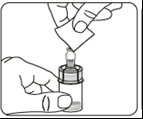 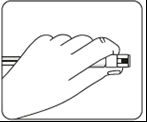  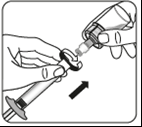 | |||
 | |||
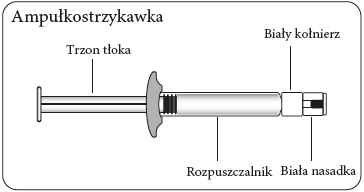
| Wipe the connection point Hold the vial vertically to prevent leakage. Holding the vial by the base, wipe the adapter connection point (blue ring) with an alcohol-soaked swab and let it dry before connecting the syringe. Do not shake. Do not touch the exposed Luer connection point in the adapter. This will contaminate it. | Hold the syringe correctly Hold the syringe by the white collar at the end. Do not hold the glass cylinder during connection. | Remove the nozzle Holding the white collar, break off the white nozzle. Do not unscrew or cut off the white nozzle. Do not touch the syringe tip. This will contaminate it. The broken nozzle can be discarded.  | Connect the syringe to the adapter Hold the adapter firmly by the shield. Holding the syringe by the white collar, insert and press its end into the blue ring of the vial connector and twist to the right to secure the syringe connection to the adapter (avoid over-tightening). Do not hold the glass cylinder of the syringe. This may cause the white collar to loosen or detach. |
Step 2 Preparing the suspension
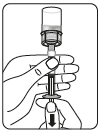
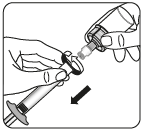
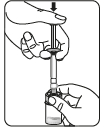
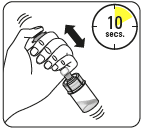
Inject the solvent
Inject all the solvent contained in the syringe into the vial.
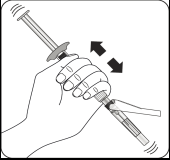
Mix the powder with the solvent
Holding the piston down, shake vigorously for at least 10 seconds, as shown.
Inspect the suspension.
A properly mixed suspension will be homogeneous, thick and milky in color.
The powder will be visible in the liquid.
Immediately proceed to the next step, so that the suspension does not sediment.
Transfer the suspension to the syringe
Turn the vial upside down.
Slowly pull the piston to draw the entire volume of the suspension from the vial into the syringe.
Remove the vial adapter
Holding the syringe by the white collar, unscrew the syringe from the adapter.
Remove both the vial and the adapter in the proper manner.

Step 3 Attaching the needle
Choose the correct needle
Choose a needle depending on the injection site (in the gluteal or deltoid muscle).
Attach the needle
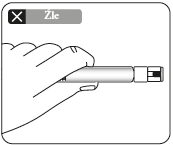
Partially tear off the blister pack and grasp the base of the needle, as shown.
Holding the syringe by the white
collar, connect it to the Luer socket in the needle screwing it in the direction of the clock hands, until the connection is secure.
Do nottouch the Luer socket in the needle. This will contaminate it.
Shake the suspension again
Completely remove the blister pack.
Immediately before injection, shake the syringe again, as sediment may appear.
Step 4 Injecting the dose
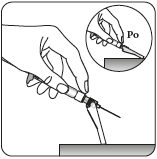
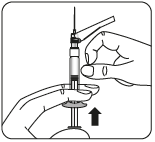
Remove the transparent needle shield
Bend the needle guard towards the syringe, as shown.
Holding the syringe by the white collar, remove the transparent needle shield.
Do nottwist the transparent needle shield, as the Luer connection may loosen.
Remove air bubbles
Holding the syringe with the needle up, gently tap to move the air bubbles to the syringe outlet.
Remove the air by carefully and slowly pressing the piston.
Inject
Immediately inject the entire contents of the syringe into the chosen muscle (gluteal or deltoid) of the patient (intramuscularly – im.).
Gluteal muscle injections should be administered in the upper outer quadrant of the buttock.
Do not administer intravenously.
Secure the needle
Holding the syringe with one hand, place the needle guard against a flat hard surface at a 45-degree angle. Press the needle into the guard with a quick, firm motion, so that it is completely enclosed.
Avoid needle stick injury:
Do notuse two hands.
Do notdeliberately remove the needle guard or handle it improperly.
Do notattempt to straighten the needle or put the guard back on if the needle is bent or damaged.
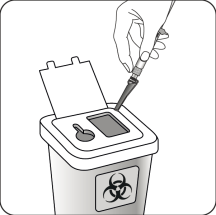
Dispose of properly
Check that the needle is completely enclosed in the shield.
Dispose of it in a special container for used medical devices.
Also dispose of the second, unused needle provided in the packaging.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterPharmathen International S.A. Pharmathen S.A. PLIVA Hrvastka d.o.o
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Risperidone TevaDosage form: Solution, 1 mg/mlActive substance: risperidonePrescription requiredDosage form: Tablets, 1 mgActive substance: risperidoneManufacturer: Orion Corporation Orion CorporationPrescription requiredDosage form: Tablets, 2 mgActive substance: risperidoneManufacturer: Orion Corporation Orion CorporationPrescription required
Alternatives to Risperidone Teva in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Risperidone Teva in Іспанія
Alternative to Risperidone Teva in Україна
Online doctors for Risperidone Teva
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Risperidone Teva – subject to medical assessment and local rules.









