
Rhesonativ

Ask a doctor about a prescription for Rhesonativ

How to use Rhesonativ
Package Leaflet: Information for the User
Rhesonativ, 750 IU/ml, Solution for Injection
Human Anti-D Immunoglobulin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
Keep this package leaflet, you may need to read it again.
In case of any doubts, consult a doctor, pharmacist, or nurse.
This medicine has been prescribed to you personally; do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
If you experience any side effects, including those not listed in this package leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Rhesonativ and what is it used for
- 2. Important information before using Rhesonativ
- 3. How to use Rhesonativ
- 4. Possible side effects
- 5. How to store Rhesonativ
- 6. Contents of the package and other information
1. What is Rhesonativ and what is it used for
Rhesonativ is an immunoglobulin and contains antibodies against the Rh factor. If a woman who does not have the Rh factor on her red blood cells (Rh negative) is pregnant and her unborn child has the Rh factor (Rh positive), her immune system may be stimulated to produce antibodies against the Rh factor. These antibodies can harm the unborn child, especially in subsequent pregnancies.
Rhesonativ is used to prevent immunization of the Rh-negative woman during pregnancy and childbirth, and thus prevent damage to the unborn child. Rhesonativ is used in Rh-negative women in the following cases:
Preventive therapy for serological conflict in pregnant women with negative Rh;
Childbirth of an Rh-positive child;
Miscarriage/threatened miscarriage;
Ectopic pregnancy, certain pathologies in the uterus (molar pregnancy) or in case of bleeding and fetal blood entering the isolated maternal circulation or fetal death in the later stages of pregnancy;
Invasive procedures during pregnancy, such as amniocentesis, cordocentesis, biopsy, or manipulative obstetric procedures, e.g., manual rotation of the fetus to improve its position in the uterus, or after abdominal trauma, as well as intrauterine surgical procedures on the fetus.
Rhesonativ may also be used in Rh-negative patients who have been mistakenly transfused with Rh-positive blood.
2. Important information before using Rhesonativ
When not to use Rhesonativ:
if you are allergic to human normal immunoglobulin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Rhesonativ, discuss it with your doctor, pharmacist, or nurse.
Tell your doctor about any other illnesses.
Rhesonativ is not indicated for administration to Rh (D) positive individuals or to individuals who have been previously immunized with the Rh (D) antigen.
Actual hypersensitivity reactions (allergic reactions) are rare but may occur.
In case of suspected allergy or severe allergic reaction (anaphylactic reaction), inform your doctor or nurse immediately. Symptoms of these reactions include, for example, dizziness, rapid heartbeat, low blood pressure, difficulty breathing and swallowing, chest tightness, itching, generalized urticaria, swelling of the face, tongue, or throat, collapse, and rash. If you experience any of these symptoms, seek medical help immediately.
If you experience symptoms such as shortness of breath, pain, and swelling of a limb or chest pain, contact your doctor or nurse immediately, as these may be signs of a blood clot.
Children
There are no available data on the use of the medicine in children.
Overweight patients
In the case of overweight/obese patients, consider using an intravenous anti-D product.
Virus safety
For medicines derived from human blood or plasma, methods are used to prevent the transmission of infection to the patient. These include:
careful selection of blood and plasma donors to exclude individuals who may be carriers of infection,
testing of individual donations and plasma pools for the presence of viruses/infections,
use of virus inactivation or removal methods in the processing of blood or plasma.
Despite these methods, when using medicines derived from human blood or plasma, it is not possible to completely eliminate the risk of transmission of infection. This also applies to unknown or new viruses or other types of infection.
The methods used appear to be effective against enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C virus, and for non-enveloped hepatitis A virus.
These methods may have limited effectiveness against non-enveloped viruses, such as parvovirus B19.
The use of immunoglobulin products is not associated with the occurrence of hepatitis A or parvovirus B19 infection, possibly due to the presence of protective antibodies against these infections in these products.
In any case, when using Rhesonativ, it is recommended to record the name and batch number of the product for identification of the batches used.
Rhesonativ and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
Rhesonativ may reduce the effectiveness of live vaccines, such as measles, mumps, rubella, and chickenpox vaccines. This applies to vaccines administered approximately 2-4 weeks before and after administration of any anti-D injection. After administration of Rhesonativ, vaccination with these vaccines should be performed after 3 months. Therefore, inform the doctor who intends to perform the vaccination about the use of Rhesonativ.
In the case of blood sampling for laboratory tests, inform your doctor about the use of immunoglobulins, as this treatment may affect laboratory test results.
Pregnancy and breastfeeding
Rhesonativ is intended for use during pregnancy and may be used during breastfeeding.
Driving and using machines
No effect on the ability to drive and use machines has been observed.
Rhesonativ contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per 1 ml (750 IU), which means that the medicine is considered "sodium-free".
3. How to use Rhesonativ
The need to use Rhesonativ and its dose will be decided by your doctor. Rhesonativ is administered by intramuscular injection by medical personnel.
4. Possible side effects
Like all medicines, Rhesonativ can cause side effects, although not everybody gets them.
The frequency of the following side effects is unknown (frequency cannot be estimated from the available data): headache, rapid heartbeat, low blood pressure, wheezing, vomiting, nausea, skin reactions, joint pain, lower back pain, dizziness, fever, feeling of discomfort, including discomfort in the chest, chills, injection site reactions, such as swelling and pain, decrease in red blood cell count, and acute allergic reactions, including anaphylactic shock.
In case of symptoms of anaphylactic shock, such as dizziness, nausea, vomiting, stomach cramps, cough, difficulty breathing and swallowing, cyanosis, itching, urticaria, rash, rapid heartbeat, low blood pressure, swelling of the face, tongue, or throat, collapse, or chest pain, seek medical help immediately.
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
- 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, e-mail: Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Rhesonativ
Keep the medicine out of the sight and reach of children.
Store in a refrigerator (2°C – 8°C). Do not freeze. Store the ampoules in the outer packaging to protect from light.
Within the shelf life, the product can be stored at a temperature below 25°C for up to one month without the need to return it to the refrigerator, but it must be discarded if not used within this time.
Do not use this medicine after the expiry date stated on the label and outer packaging after: EXP (abbreviation used to describe the expiry date). The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the package and other information
What Rhesonativ contains
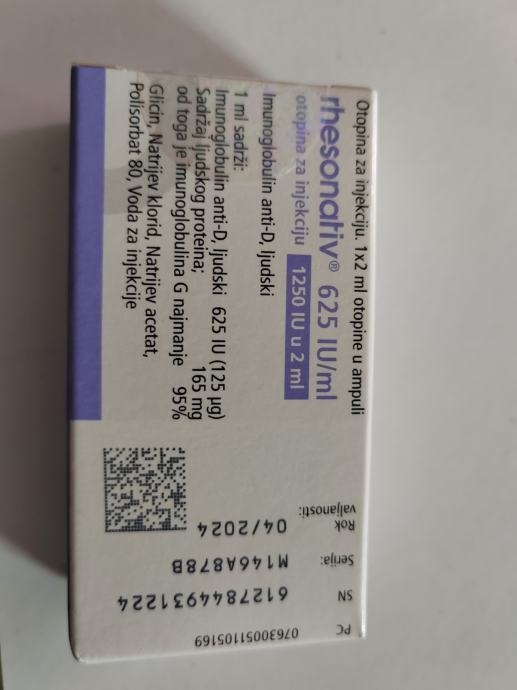
- The active substance is human anti-D immunoglobulin. 1 ml contains 750 IU (150 micrograms) of human anti-D immunoglobulin.
- One ampoule of 1 ml contains 750 IU (150 micrograms) of human anti-D immunoglobulin. One ampoule of 2 ml contains 1500 IU (300 micrograms) of human anti-D immunoglobulin.
- The protein content is 165 mg, of which at least 95% is immunoglobulin G.
- The other ingredients are: glycine, sodium chloride, sodium acetate, polysorbate 80, and water for injections.
What Rhesonativ looks like and contents of the package
Rhesonativ is a solution for injection (750 IU/ml or 1500 IU/ml in an ampoule).
Package sizes: 1 ampoule of 1 ml and 1 ampoule of 2 ml.
The solution may appear colorless, pale yellow, or light brown.
Not all package sizes may be marketed.
Marketing authorization holder and manufacturer
Octapharma (IP) SPRL
65 Allée de la Recherche
1070 Anderlecht
Belgium
Manufacturer
Octapharma AB
Lars Forssells gata 23
112 75 Stockholm
Sweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Member State | Medicinal product name |
| Bulgaria | Rhesonativ 750 IU/ml, solution for injection |
| Poland | Rhesonativ, 750 IU/ml, solution for injection |
| Portugal | Rhesonativ 750 IU/ml, injectable solution |
| Romania | Rhesonativ 750 IU/ml, injectable solution |
| Slovakia | Rhesonativ 750 IU/ml, injection solution |
| Slovenia | Rhesonativ 750 IU/ml, solution for injection |
| Sweden | Rhesonativ, 750 IU/ml, injectable solution |
| Hungary | Rhesonativ 750 IU/ml, solution for injection |
Date of last revision of the package leaflet: 24.05.2021
Information intended for healthcare professionals only:
Before use, the product should be brought to room temperature or body temperature.
Do not use a non-homogeneous solution or one that contains sediment.
The contents of the ampoule should be used immediately after opening. Unused medicinal product or waste materials should be disposed of in accordance with local requirements.
Rhesonativ must be administered intramuscularly. Before injection, withdraw the plunger of the syringe to ensure that the needle is not in a blood vessel.
In situations where intramuscular administration is contraindicated (coagulation disorders), the injection can be administered subcutaneously if an intravenous product is not available. In this case, gentle pressure should be applied using a swab at the injection site.
If larger volumes are required (more than 2 ml in children and more than 5 ml in adults), it is recommended to administer them in divided doses at different sites.
This medicinal product must not be mixed with other medicinal products.
In the case of overweight/obese patients, consider using an intravenous anti-D product due to possible lack of efficacy with intramuscular administration.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOctapharma AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RhesonativDosage form: Solution, 150 mcg/mlActive substance: anti-D (rh) immunoglobulinManufacturer: Synthaverse S.A.Prescription requiredDosage form: Solution, 50 mcg/mlActive substance: anti-D (rh) immunoglobulinManufacturer: Synthaverse S.A.Prescription requiredDosage form: Solution, 625 IU/ml (125 mcg)Active substance: anti-D (rh) immunoglobulinManufacturer: Octapharma ABPrescription required
Alternatives to Rhesonativ in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Rhesonativ in Ukraine
Alternative to Rhesonativ in Spain
Online doctors for Rhesonativ
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Rhesonativ – subject to medical assessment and local rules.














