

Relanium

Ask a doctor about a prescription for Relanium

How to use Relanium
Leaflet accompanying the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Relanium
5 mg, tablets
Diazepam
It is necessary to carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- The leaflet should be kept so that it can be re-read if necessary.
- In case of any doubts, the doctor or pharmacist should be consulted.
- This medicine has been prescribed to a specific person. It should not be passed on to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Relanium and what is it used for
- 2. Important information before taking Relanium
- 3. How to take Relanium
- 4. Possible side effects
- 5. How to store Relanium
- 6. Contents of the packaging and other information
1. What is Relanium and what is it used for
Relanium contains the active substance diazepam, which belongs to a group of medicines called benzodiazepine derivatives.
The medicine has anxiolytic, sedative, hypnotic, and anticonvulsant effects. It also reduces skeletal muscle tension.
Indications for use:
- Treatment of anxiety states.
- Treatment of insomnia associated with anxiety states (only in cases of severe disorders that prevent normal daily activities or cause extreme discomfort to the patient).
- Control of muscle spasms, including those associated with increased muscle tension of central origin.
- Pharmacological preparation of the patient before minor surgical procedures (premedication).
- Treatment of symptoms of acute alcohol withdrawal.
2. Important information before taking Relanium
When not to take Relanium
- if the patient is allergic (hypersensitive) to the active substance, other benzodiazepines, or any of the other ingredients of the medicine (listed in section 6),
- if the patient has excessive muscle weakness (myasthenia gravis),
- if the patient has severe respiratory failure,
- if the patient has sleep apnea syndrome (repeated pauses in breathing during sleep, leading to hypoxia),
- if the patient has severe liver failure.
Page 1 8
Warnings and precautions
Before starting to take Relanium, this should be discussed with the doctor.
- Diazepam is not recommended for the treatment of chronic psychoses or states with phobias or obsessions.
- Relanium should not be taken with alcohol, sleeping pills, or sedatives, as it may enhance their effects (see section: Relanium and other medicines).
- If the patient is addicted to alcohol or drugs, Relanium should be avoided. This does not apply to the treatment of acute withdrawal symptoms. In such cases, the doctor will regularly monitor the patient and recommend the use of the smallest effective dose.
- If the patient has a history of alcohol or drug abuse, Relanium should be used with caution.
- If Relanium is used for several weeks, tolerance to the medicine may occur (reduced efficacy of the medicine).
- Long-term use of Relanium, especially in high doses, may lead to physical and psychological dependence. The risk of dependence increases with the dose and duration of treatment. It is higher in patients who have previously abused alcohol or drugs and in patients with personality disorders.
- If the patient is dependent on Relanium, sudden withdrawal of the medicine may cause withdrawal symptoms, such as headaches, muscle pain, extreme anxiety, tension, agitation, confusion, irritability. In severe cases, the following symptoms may occur: loss of reality, personality disorders, numbness and tingling of limbs, hallucinations, seizures.
- The occurrence of withdrawal symptoms may also be caused by switching from Relanium to benzodiazepines with a short duration of action.
- During withdrawal or after withdrawal of Relanium, a transient increase in the symptoms of the disease for which the medicine was used may occur. To prevent this, the medicine should be withdrawn gradually, strictly according to the doctor's instructions (see section: Stopping Relanium).
- Relanium may cause anterograde amnesia (inability to learn and remember new information, see section 4: Possible side effects). To reduce the risk of anterograde amnesia, it is essential that the patient has uninterrupted sleep for 7 to 8 hours.
- In children and the elderly, there is a higher risk of adverse psychological reactions and paradoxical reactions (see section 4: Possible side effects).
- In elderly patients and weakened patients, the doctor may recommend a lower dose of the medicine (see section 3: How to take Relanium). The medicine reduces muscle tension. This increases the risk of falls and, consequently, hip fractures in the elderly.
- If the patient has chronic respiratory failure, the doctor will use a lower dose of the medicine, as respiratory disorders may occur.
- In patients with heart failure, caution should be exercised when using Relanium.
- In patients with epilepsy, sudden discontinuation of treatment should be avoided due to the risk of seizures.
- If the patient has severe liver failure, Relanium should not be used due to the risk of neurological disorders (hepatic encephalopathy).
- In patients with impaired liver and/or kidney function, the doctor will decide on a dose reduction. In the case of long-term use of Relanium, the doctor may order blood tests to closely monitor the number of blood cells and liver function.
- If the patient has psychotic disorders (e.g., schizophrenia), Relanium should not be used.
- If the patient has depression or anxiety states associated with depression, Relanium should not be used as the only medicine, as it may increase the risk of suicide.
- The use of Relanium in people who are grieving (after the loss of loved ones) does not improve their well-being. Page 2 8
Children and adolescents
Use of Relanium in children; the doctor will determine the dose and duration of treatment (see section 3: How to take Relanium).
Relanium is not recommended for the treatment of anxiety states and insomnia in children.
The medicine should not be used in children under 6 years of age, as the safety and efficacy of the medicine have not been assessed in this age group. Only when it is impossible to use alternative medicines, the doctor may decide to administer Relanium, but under close supervision of a specialist (pediatrician, neurologist, psychiatrist, anesthesiologist, and intensive care specialist), who will determine the dose.
Relanium and other medicines
The patient should tell the doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Particularly, the patient should inform the doctor about the following medicines, as they may enhance the effects of Relanium:
- atazanavir and ritonavir - used in the treatment of HIV and hepatitis C,
- cimetidine - a medicine used in the treatment of stomach and duodenal ulcers,
- ketoconazole - a medicine used in the treatment of fungal infections,
- medicines used in the treatment of depression, such as fluvoxamine, fluoxetine,
- omeprazole - a medicine that reduces stomach acid,
- cisapride - a medicine used in the treatment of gastroesophageal reflux disease,
- disulfiram - a medicine used in the treatment of alcoholism,
- isoniazid - a medicine used in the treatment of tuberculosis,
- propranolol - a medicine used in the treatment of high blood pressure and irregular heartbeat,
- rifampicin - a medicine used in the treatment of bacterial infections,
- antipsychotic medicines,
- anxiolytic and sedative medicines,
- sleeping pills,
- medicines used in the treatment of epilepsy,
- medicines used for general anesthesia,
- antihistamines with sedative effects - used in the treatment of allergies,
- opioid analgesics. Concomitant use of Relanium and opioids (strong painkillers, medicines used in substitution therapy for addiction, and some cough medicines) increases the risk of drowsiness, breathing difficulties (respiratory depression), coma, and can be life-threatening. Therefore, concomitant use of these medicines can only be considered when other treatment options are not possible. If the doctor has prescribed Relanium concomitantly with opioids, they should limit the dose and duration of concomitant treatment. The patient should inform the doctor about all opioid medicines they are taking and strictly follow the dosage instructions. It may be helpful to inform friends or relatives to be aware of the above symptoms. In case of such symptoms, the patient should contact their doctor.
Relanium may affect the efficacy of phenytoin used as an antiepileptic medicine.
Theophylline - a medicine used in the treatment of asthma - may reduce the efficacy of benzodiazepines.
Concomitant use of buprenorphine with Relanium (or other benzodiazepines) should be avoided, as it may cause death due to respiratory depression.
Concomitant use of valproic acid increases the risk of psychosis.
Relanium with food, drink, and alcohol
During treatment with this medicine, the patient should not drink any alcoholic beverages. Alcohol enhances the sedative effect of Relanium.
Page 3 8
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before taking this medicine.
Relanium should not be used during pregnancy, especially in the first and third trimesters.
Use of Relanium during pregnancy is only allowed when the doctor considers it absolutely necessary and will closely monitor the patient.
If a woman takes Relanium in the last three months of pregnancy or during childbirth, the newborn may experience hypothermia, decreased muscle tone, irregular heartbeat, difficulty sucking, and breathing difficulties.
In children of mothers who take diazepam for a long time in late pregnancy, physical dependence and withdrawal symptoms may develop after birth.
The medicine passes into breast milk, so it should not be used during breastfeeding.
If the patient is breastfeeding, they should consult their doctor before starting treatment with Relanium.
Driving and using machines
Relanium may impair psychophysical fitness. The patient should not drive vehicles or operate machines while taking this medicine.
Relanium contains lactose monohydrate.
If the patient has previously been diagnosed with intolerance to some sugars, they should consult their doctor before taking the medicine.
Relanium contains sodium (present in quinoline yellow (E 104)).
The medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means it is considered "sodium-free".
Relanium contains quinoline yellow (E 104).
Due to the presence of quinoline yellow, the medicine may cause allergic reactions.
3. How to take Relanium
Relanium should always be taken according to the doctor's instructions. In case of doubts, the patient should consult their doctor or pharmacist.
Treatment with diazepam should last as short as possible and not longer than 4 weeks in the case of insomnia or 8-12 weeks in the case of anxiety states, including the time of gradual withdrawal of the medicine. The treatment time should not be extended beyond the above periods without re-evaluation of the patient's condition by the doctor.
Dosage in adults
- anxiety states and insomnia
- anxiety states: the usual dose is 2 mg three times a day; the maximum dose used in the treatment of anxiety states is 30 mg per day in divided doses.
- insomnia associated with anxiety states: 5 mg to 15 mg per day, before sleep. The doctor will recommend the smallest dose that controls the symptoms;
- states associated with muscle spasms
- muscle spasms: 2 mg to 15 mg per day in divided doses.
- control of increased muscle tension of central origin: 2 mg to 60 mg per day in divided doses.
- preparation (premedication) for general anesthesia
- 5 mg to 20 mg.
- treatment of symptoms of acute alcohol withdrawal
- usually 10 mg, 3 to 4 times within the first 24 hours, then the doctor may reduce the dose to 5 mg, 3 to 4 times a day.
Dosage in children
- anxiety states and insomnia
- Relanium is not recommended for the treatment of anxiety states and insomnia in children.
- states associated with muscle spasms
- in the control of irritability and increased muscle tension of central origin: 5 mg to 40 mg per day in divided doses.
- children from 6 to 12 years: the initial dose is 5 mg twice a day.
- children from 12 to 18 years: the initial dose is 10 mg twice a day; the maximum dose is 40 mg per day.
- preparation (premedication) for general anesthesia
- children from 6 to 18 years: 2 mg to 10 mg.
Dosage in children under 6 years
Relanium tablets are not intended for children under 6 years of age.
Dosage in elderly and weakened patients
The dose should not exceed half of the usual dose used in adults.
Patients with impaired renal and/or hepatic function
In patients with impaired renal and/or hepatic function, the doctor will decide on a dose reduction.
Method of administration
- The medicine should be taken orally.
- The medicine should not be taken for longer than 4 weeks in the case of insomnia or 8 to 12 weeks in the case of anxiety states (including the time of gradual withdrawal of the medicine).
Use of a higher than recommended dose of Relanium
In case of taking a higher dose than recommended, the patient should immediately seek medical help or go to the nearest hospital.
Overdose of Relanium rarely poses a threat to life if the medicine was taken alone.
The following overdose symptoms may occur: drowsiness, clumsiness, weakness, slurred speech, nystagmus, areflexia (loss of reflexes), apnea, hypotension, circulatory and respiratory depression, and coma.
Coma, if it occurs, usually lasts a few hours, but may last longer and occur at regular intervals, especially in the elderly.
Missing a dose of Relanium
In case of missing a dose, the patient should take the medicine as soon as possible. If it is almost time for the next dose, the patient should skip the missed dose. The next dose should be taken according to the doctor's instructions. The patient should not take a double dose to make up for the missed dose.
Stopping Relanium
The medicine should be withdrawn gradually. Sudden discontinuation of Relanium may cause a transient increase in the symptoms of the disease for which the medicine was used. Other symptoms may also occur, such as headaches, muscle pain, extreme anxiety, tension, agitation, confusion, irritability. In severe cases, the following symptoms may occur: loss of reality, personality disorders, hypersensitivity to sound, numbness and tingling of limbs, hallucinations, seizures.
The patient should always follow the doctor's instructions. In case of any doubts related to the use of the medicine, the patient should consult their doctor or pharmacist.
Page 5 8
4. Possible side effects
Like all medicines, Relanium can cause side effects, although not everybody gets them.
The most common side effects are:
- fatigue,
- drowsiness,
- muscle weakness. These effects occur especially at the beginning of treatment and disappear after repeated administration.
Frequency not known (cannot be estimated from the available data):
- paradoxical reactions: anxiety, agitation, irritability, aggressive behavior, anger, rage, delusions,
- nightmares, hallucinations, psychoses,
- changes in behavior, including inadequate behavior,
- disorders of thought, disorientation regarding time, place, situation, or own person (confusion),
- apathy (emotional flattening),
- reduced alertness,
- depression,
- decreased or increased libido,
- clumsiness (difficulty maintaining balance) - may cause falls and dangerous fractures, especially in the elderly,
- slurred speech, unclear speech,
- headaches, dizziness,
- tremors,
- inability to learn and remember new information (anterograde amnesia); to prevent this, the patient should have uninterrupted sleep for 7 to 8 hours,
- double vision, blurred vision,
- heart failure, cardiac arrest, irregular heartbeat,
- hypotension, bradycardia,
- slow and shallow breathing, apnea,
- nausea, constipation, gastrointestinal disorders (e.g., bloating),
- dry mouth or excessive salivation,
- jaundice (yellowing of the skin and whites of the eyes), increased activity of liver enzymes (aminotransferases and alkaline phosphatase) in the blood,
- skin reactions,
- urinary incontinence or inability to urinate (strong urge to urinate, abdominal pain),
- blood disorders (blood dyscrasia). Long-term use (even at therapeutic doses) may lead to physical dependence; sudden withdrawal of the product may cause withdrawal symptoms or rebound phenomenon (see section Warnings and precautions). Psychological dependence may occur. Cases of benzodiazepine abuse have been reported.
Reporting side effects
If side effects occur, including any side effects not listed in the leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
Page 6 8
5. How to store Relanium
The medicine should be stored out of sight and reach of children.
Do not store above 25°C. Store in the original packaging to protect from light and moisture.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Relanium contains
The active substance of Relanium is diazepam. One tablet contains 5 mg of diazepam.
The other ingredients are: lactose monohydrate, potato starch, talc, magnesium stearate, anhydrous colloidal silica, quinoline yellow (E 104).
What Relanium tablets 5 mg look like and what the packaging contains
Relanium is a yellow, round, biconvex tablet with a smooth surface.
The packaging contains 20 tablets.
Removing a tablet from the blister pack
These tablets are provided in a special packaging with a child-resistant closure.
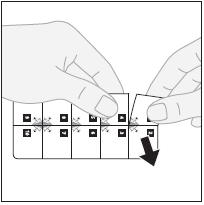
Each blister pack segment with one tablet has a number. The tablets should be taken in sequence, according to the numbering, starting from number 1.
- 1. Separating the tablet:to separate the blister pack segment with one tablet, tear the blister pack along the perforated lines.
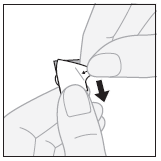
- 2. Removing the outer layer:starting from the corner, peel off and remove the outer layer from the separated blister pack segment.
Page 7 8
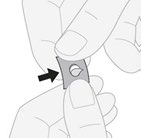
- 3. Removing the tablet:gently push the tablet through the foil.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Lithuania, the country of export:
GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland
Manufacturer:
Delpharm Poznań Spółka Akcyjna, ul. Grunwaldzka 189, 60-322 Poznań
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Polfarmex S.A., ul. Józefów 9, 99-300 Kutno
Lithuanian marketing authorization number, in the country of export: LT/1/94/1933/001
LT/1/94/1933/002
Parallel import authorization number: 23/15 Date of leaflet approval: 21.04.2023
[Information about the trademark]
Page 8 8
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)GlaxoSmithKline Trading Services Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RelaniumDosage form: Tablets, 2 mgActive substance: diazepamManufacturer: Santa S.A.Prescription requiredDosage form: Solution, 10 mg/2 mlActive substance: diazepamManufacturer: AS GrindeksPrescription not required
Alternatives to Relanium in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Relanium in Spain
Alternative to Relanium in Ukraine
Online doctors for Relanium
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Relanium – subject to medical assessment and local rules.









