

Proaxon

Ask a doctor about a prescription for Proaxon

How to use Proaxon
Package Leaflet: Information for the User
Proaxon
1000 mg/10 ml, Oral Solution
Cytidine
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Proaxon and what is it used for
- 2. Important information before taking Proaxon
- 3. How to take Proaxon
- 4. Possible side effects
- 5. How to store Proaxon
- 6. Contents of the pack and other information
1. What is Proaxon and what is it used for
Proaxon contains the active substance citidine, which belongs to a group of medicines called psychostimulants and nootropics (so-called "memory enhancers"). These medicines work by improving brain function.
Proaxon is used to treat neurological and cognitive disorders caused by:
- interruptions in blood supply to the brain due to the formation of a blood clot or damage to blood vessels in the brain (e.g., stroke);
- head injuries (e.g., resulting from a blow).
2. Important information before taking Proaxon
When not to take Proaxon:
- if you are allergic to citidine or any of the other ingredients of this medicine (listed in section 6);
- if you have increased sympathetic nervous system tone, which is a serious disease characterized by low blood pressure, excessive sweating, rapid heartbeat, and fainting.
Warnings and precautions
Before taking Proaxon, discuss with your doctor:
- if you are allergic to acetylsalicylic acid, as this medicine may cause asthma attacks.
Children and adolescents
There is a lack of sufficient data on the use of Proaxon in children, therefore this medicine should only be given to children when a doctor decides it is necessary.
Proaxon and other medicines
Tell your doctor about all the medicines you are taking or have recently taken, as well as any medicines you plan to take.
Citidine enhances the effects of L-dopa (also known as levodopa), so concurrent use of these medicines should not be undertaken without prior consultation with a doctor.
L-dopa is used to treat Parkinson's disease.
Concomitant use of citidine with meclofenoxate, a medicine that stimulates brain activity, is not recommended.
Proaxon with food and drink
Proaxon can be taken on an empty stomach or with food.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, or think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Proaxon should not be taken if you are pregnant, think you may be pregnant, or are breastfeeding, unless your doctor considers it necessary.
Driving and using machines
Proaxon has no effect on the ability to drive and use machines.
This medicine may cause asthma attacks, especially in people allergic to acetylsalicylic acid.
Proaxon contains sorbitol (E420). The medicine contains 2000 mg of sorbitol in each sachet, which corresponds to 200 mg/ml. If you have previously been diagnosed with intolerance to some sugars, you should consult your doctor before taking this medicine.
Proaxon contains propyl parahydroxybenzoate (E216), methyl parahydroxybenzoate
(E218) and cochineal red (E124). The medicine may cause allergic reactions (possible late reactions).
The medicine contains 80.83 mg of sodium(the main component of common salt) in each sachet, which corresponds to 4.04% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to take Proaxon
Take this medicine always as directed by your doctor. If you are unsure, ask your doctor or pharmacist.
The recommended dose is 1 to 2 sachets per day, depending on the severity of the disease symptoms.
This medicine can be taken directly or after dissolving in 120 ml of water (about half a glass of water), during or between meals.
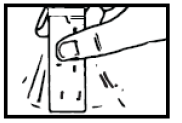
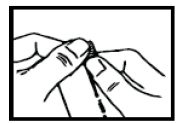
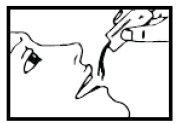
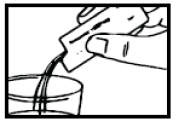
- 1. Hold the Proaxon 1000 mg sachet and shake it well.
- 2. With your other hand, tear the sachet along the perforated line.
- 3. You can take the medicine directly or
- 4. after dissolving it in half a glass of water (120 ml).
Taking more than the recommended dose of Proaxon
If you take more than the recommended dose, contact your doctor immediately.
Take the medicine packaging or this leaflet with you, so that medical personnel know what medicine has been taken.
Missing a dose of Proaxon
Take the dose as soon as possible.
Do not take a double dose to make up for the missed dose.
Stopping treatment with Proaxon
Your doctor will decide how long you should take Proaxon. Do not stop treatment without consulting your doctor.
If you have any further questions about taking this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Proaxon can cause side effects, although not everybody gets them.
Side effects that occur very rarely(less than 1 in 10,000 people):
- hallucinations,
- headaches, dizziness,
- changes in blood pressure (increased or decreased blood pressure),
- breathing difficulties (dyspnea),
- nausea, vomiting, diarrhea,
- flushing, hives, rash, petechiae,
- edema,
- chills.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181 C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Proaxon
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and sachets.
The expiry date refers to the last day of the month stated.
There are no special precautions for storage.
Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines no longer required.
This will help protect the environment.
6. Contents of the pack and other information
What Proaxon contains
- The active substance of the medicine is citidine. One milliliter of the solution contains 100 mg of citidine (as sodium salt). Each sachet (10 ml solution) contains 1000 mg of citidine (as sodium salt).
- The other ingredients are: liquid sorbitol, non-crystallizing (E420), glycerol, propyl parahydroxybenzoate (E216), methyl parahydroxybenzoate (E218), potassium sorbate, sodium saccharin, glycerol formal, cochineal red (E124), citric acid, sodium citrate, strawberry flavor, purified water.
What Proaxon looks like and contents of the pack
Proaxon is a clear, pink oral solution with a strawberry flavor and aroma.
The medicine is packaged in sachets made of PET/PX/Aluminum/RT foil containing 10 ml of solution, placed in a cardboard box.
The pack contains 10 sachets of 10 ml solution each.
Marketing authorization holder
Biofarm Sp. z o.o.
ul. Wałbrzyska 13
60-198 Poznań
tel.: +48 61 66 51 500
fax: +48 61 66 51 505
e-mail: [email protected]
Manufacturer
SAG MANUFACTURING, S.L.U
Crta. N-I, Km 36
San Agustin de Guadalix
28750 Madrid
Spain
Galenicum Health, S.L.U.
Sant Gabriel, 50
Esplugues de Llobregat
08950 Barcelona
Spain
Date of last revision of the leaflet:31.08.2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGalenicum Health S.L.U. SAG Manufacturing S.L.U.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ProaxonDosage form: Solution, 1000 mg/10 mlActive substance: citicolineManufacturer: Galenicum Health, S.L.U. SAG Manufacturing S.L.U.Prescription requiredDosage form: Tablets, 1200 mgActive substance: piracetamManufacturer: Biofarm Sp. z o.o.Prescription requiredDosage form: Tablets, 800 mgActive substance: piracetamManufacturer: Biofarm Sp. z o.o.Prescription required
Alternatives to Proaxon in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Proaxon in Spain
Alternative to Proaxon in Ukraine
Online doctors for Proaxon
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Proaxon – subject to medical assessment and local rules.











