

Pralex

Ask a doctor about a prescription for Pralex

How to use Pralex
Package Leaflet: Information for the User
Pralex, 5 mg, coated tablets
Pralex, 10 mg, coated tablets
Pralex, 15 mg, coated tablets
Pralex, 20 mg, coated tablets
Escitalopram
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet:
- 1. What is Pralex and what is it used for
- 2. Important information before taking Pralex
- 3. How to take Pralex
- 4. Possible side effects
- 5. How to store Pralex
- 6. Contents of the pack and other information
1. What is Pralex and what is it used for
Escitalopram belongs to a group of antidepressant medicines called selective serotonin reuptake inhibitors (SSRIs).
These medicines work on the serotonin system in the brain by increasing the levels of serotonin.
Disorders of the serotonin system are thought to be a major factor in the development of depression and related disorders.
Pralex contains the active substance escitalopram and is used to treat depression (major depressive episodes), anxiety disorders (such as panic disorder with or without agoraphobia, social anxiety disorder, generalized anxiety disorder, and obsessive-compulsive disorder).
Improvement may not be seen until after several weeks of treatment. You should continue to take Pralex even if you do not feel better immediately.
Tell your doctor if you do not feel better or feel worse.
2. Important information before taking Pralex
When not to take Pralex
- if you are allergic to escitalopram or any of the other ingredients of this medicine (listed in section 6)
- if you are taking other medicines that belong to a group called non-selective monoamine oxidase inhibitors (MAOIs), including selegiline (used to treat Parkinson's disease), moclobemide (used to treat depression), and linezolid (an antibiotic)
- if you have been diagnosed with a heart rhythm disorder or have a history of such a condition (identified on an ECG - a test used to evaluate heart function)
- if you are taking medicines for heart rhythm disorders or medicines that may affect heart rhythm (see also section 2 "Pralex and other medicines")
Warnings and precautions
You should talk to your doctor or pharmacist before taking Pralex.
Tell your doctor if you have any other conditions or diseases, as your doctor should take this into account.
In particular, tell your doctor:
- if you have epilepsy. If you experience seizures for the first time or if their frequency increases, you should stop taking Pralex (see also section 4 "Possible side effects")
- if you have liver or kidney disease. Your doctor may need to adjust your dose
- if you have diabetes. Taking Pralex may affect your blood sugar control. You may need to adjust your insulin dose and/or oral hypoglycemic medication
- if you have low sodium levels in your blood
- if you have a tendency to easy bruising, have a history of bleeding disorders, or are pregnant (see "Pregnancy, breastfeeding, and fertility")
- if you are undergoing electroconvulsive therapy
- if you have coronary heart disease
- if you currently have or have a history of heart disease or have had a heart attack
- if you have a slow heart rate at rest and/or may have low levels of potassium or magnesium in your blood due to severe diarrhea or vomiting (with nausea) or due to the use of diuretics
- if you have a fast or irregular heartbeat, fainting, collapse, or dizziness when standing up, which may be symptoms of abnormal heart rhythm
- if you have or have had eye problems, such as certain types of glaucoma (increased pressure in the eye)
Medicines like Pralex (SSRIs or SNRIs) may cause symptoms of sexual dysfunction (see section 4). In some cases, these symptoms have persisted after stopping treatment.
Caution:
In some patients with bipolar affective disorder, a manic phase may occur. This is characterized by unusual and rapidly changing ideas, unjustified feelings of happiness, and excessive physical activity. If you experience these symptoms, you should contact your doctor.
In the first few weeks of treatment, you may also experience symptoms such as restlessness or difficulty sitting or standing still. If you experience these symptoms, you should contact your doctor or pharmacist immediately.
Suicidal thoughts, worsening of depression or anxiety disorders
People with depression or anxiety disorders may sometimes have thoughts of self-harm or suicide. These symptoms or behaviors may worsen when taking antidepressant medicines, as these medicines start to work usually after 2 weeks, sometimes later.
Suicidal thoughts, self-harm, or suicidal behavior are more likely to occur if:
- you have had suicidal thoughts or self-harm in the past
- you are a young adult; clinical trial data suggest an increased risk of suicidal behavior in people under 25 years of age with mental health disorders who are being treated with antidepressant medicines
If you experience suicidal thoughts or self-harm, you should contact your doctor or go to the hospital immediately.
It may be helpful to inform your relatives or friends about your depression or anxiety disorder and ask them to read this leaflet.You can ask your relatives or friends to tell you if they notice that your depression or anxiety has worsened or if you have any worrying changes in your behavior.
Children and adolescents under 18 years of age
Pralex should not be used in children and adolescents under 18 years of age. It is also important to note that in patients under 18 years of age, taking medicines of this class may increase the risk of side effects, such as suicidal attempts, suicidal thoughts, and hostility (especially aggression, rebellious behavior, and expressions of anger). However, your doctor may prescribe Pralex to patients under 18 years of age if they consider it to be in their best interest. If your doctor has prescribed Pralex to a patient under 18 years of age, and you have any concerns, please contact your doctor. If a patient under 18 years of age taking Pralex develops or worsens any of the above symptoms, you should inform your doctor.
Additionally, there is currently no data on the long-term safety of Pralex in this age group regarding growth, maturation, and cognitive and behavioral development.
Pralex and other medicines
Tell your doctor or pharmacist about all the medicines you are taking, have recently taken, or plan to take.
Tell your doctor if you are taking any of the following medicines:
- non-selective monoamine oxidase inhibitors (MAOIs) containing active substances such as phenelzine, iproniazid, isocarboxazid, nialamide, and tranylcypromine (used to treat depression). If you have taken any of these medicines, you should wait 14 days before starting to take Pralex. After stopping treatment with Pralex, you should wait 7 days before taking any of these medicines.
- reversible, selective monoamine oxidase A inhibitors (MAO-A) containing moclobemide (used to treat depression)
- irreversible monoamine oxidase B inhibitors (MAO-B) containing selegiline (used to treat Parkinson's disease). These medicines increase the risk of side effects.
- the antibiotic linezolid
- lithium (used to treat bipolar affective disorders) and tryptophan
- imipramine and desipramine (used to treat depression)
- sumatriptan and similar medicines (used to treat migraine), buprenorphine, and tramadol (used to treat severe pain). These medicines may increase the risk of side effects.
- cimetidine, lansoprazole, and omeprazole (used to treat stomach ulcers), fluconazole (an antifungal medicine), fluvoxamine (an antidepressant), and ticlopidine (used to reduce the risk of stroke). These medicines may cause an increase in escitalopram levels in the blood.
- St. John's Wort (Hypericum perforatum) - a herbal medicine used to treat depression
- acetylsalicylic acid (aspirin) and non-steroidal anti-inflammatory medicines (used to treat pain or thin the blood, known as anticoagulants). These medicines may increase the risk of bleeding.
- warfarin, dipyridamole, and phenprocoumon (used to thin the blood, known as anticoagulants). Your doctor may need to monitor your blood clotting time when starting or stopping treatment with Pralex.
and after stopping treatment with Pralex to determine if the dose of the anticoagulant is still appropriate.
- mefloquine (used to treat malaria), bupropion (used to treat depression), and tramadol (used to treat severe pain) due to the possible risk of lowering the seizure threshold.
- neuroleptics (used to treat schizophrenia, psychosis) and antidepressants (tricyclics, SSRIs due to the possible risk of lowering the seizure threshold.
- flecainide, propafenone, and metoprolol (used to treat heart conditions), clomipramine and nortriptyline (antidepressants), and risperidone, thioridazine, and haloperidol (antipsychotics). Your doctor may need to adjust the dose of Pralex.
- medicines that lower potassium or magnesium levels in the blood, due to the increased risk of life-threatening heart rhythm disorders.
DO NOT TAKE PRALEXif you are taking medicines used to treat heart rhythm disorders or medicines that may affect heart rhythm, such as:
- antiarrhythmic medicines of class IA and III, antipsychotics (e.g., phenothiazine derivatives, pimozide, haloperidol), tricyclic antidepressants, certain antibiotics (e.g., sparfloxacin, moxifloxacin, erythromycin IV, pentamidine, antimalarial treatment mainly halofantrine), certain antihistamines (e.g., astemizole, hydroxyzine, mizolastine)
In case of any doubts, consult your doctor.
Pralex with food, drink, and alcohol
Pralex can be taken with or without food (see section 3 "How to take Pralex").
As with other medicines, it is not recommended to take Pralex and drink alcohol at the same time, although no interaction has been demonstrated between Pralex and alcohol.
Pregnancy, breastfeeding, and fertility
If you are pregnant, think you may be pregnant, or are planning to become pregnant, you should consult your doctor or pharmacist before taking this medicine.
Do not take Pralex during pregnancy, unless your doctor has discussed the risks and benefits of treatment with you.
Tell your midwife and/or doctor that you are taking Pralex. Taking medicines like Pralex during pregnancy, especially in the last 3 months, may increase the risk of a serious condition in newborns called persistent pulmonary hypertension of the newborn (PPHN), which causes rapid breathing and bluish discoloration of the baby's skin. These symptoms usually occur within the first 24 hours after birth. If you notice any of these symptoms in your baby, you should contact your midwife and/or doctor immediately.
If you are taking Pralex in the last 3 months of pregnancy, you should be aware that your newborn may experience the following symptoms: difficulty breathing, bluish skin, seizures, changes in body temperature, difficulty feeding, vomiting, low blood sugar, stiffness or floppiness, increased reflexes, tremors, tremors, irritability, lethargy, constant crying, and difficulty sleeping. If your newborn experiences any of these symptoms, you should contact your doctor immediately.
Additionally, there is currently no data on the long-term safety of Pralex in this age group regarding growth, maturation, and cognitive and behavioral development.
Driving and using machines
You should not drive or operate machinery until you know how Pralex affects you.
Other ingredients
The medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means it is essentially "sodium-free".
3. How to take Pralex
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Adults
Depression
The recommended dose is 10 mg, taken as one dose per day. The dose may be increased by your doctor to a maximum of 20 mg per day.
Panic disorder
The initial dose is 5 mg per day for the first week, then the dose is increased to 10 mg per day. The dose may then be increased by your doctor to a maximum of 20 mg per day.
Social anxiety disorder
The recommended dose is 10 mg, taken as one dose per day. The dose may then be decreased by your doctor to 5 mg per day or increased to a maximum of 20 mg per day, depending on your response to the medicine.
Generalized anxiety disorder or obsessive-compulsive disorder
The recommended dose of Pralex is 10 mg, taken as one dose per day. The dose may be increased by your doctor to a maximum of 20 mg per day.
Elderly patients (over 65 years of age)
The recommended initial dose is 5 mg, taken as one dose per day. The dose may be increased by your doctor to 10 mg per day.
Children and adolescents (under 18 years of age)
Pralex should not normally be used in children and adolescents. Additional information is provided in section 2 "Important information before taking Pralex".
Kidney problems
Caution should be exercised in patients with severe kidney problems. The medicine should be taken as directed by your doctor.
Liver problems
Patients with liver problems should not exceed a dose of 10 mg per day. The medicine should be taken as directed by your doctor.
Poor metabolizers of medicines that are metabolized by the CYP2C19 enzyme
Patients with this known genotype should not exceed a dose of 10 mg per day. The medicine should be taken as directed by your doctor.
How to take Pralex
Pralex can be taken with or without food. The tablets should be swallowed with water. Do not chew them, as they have a bitter taste.
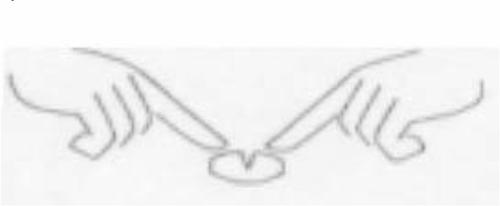
Pralex 10 mg, 15 mg, and 20 mg: If necessary, the tablets can be divided into two halves by placing the tablet on a flat surface with the groove facing upwards. The tablets can then be broken in half by pressing each end downwards with your index fingers, as shown in the picture.
Duration of treatment
You may start to feel better after a few weeks of treatment. Therefore, you should continue to take Pralex, even if it takes some time before you feel better.
Do not change your dose without consulting your doctor.
Take the medicine for as long as your doctor recommends. If you stop treatment too early, your symptoms may return. It is recommended to continue treatment for at least 6 months after you have recovered.
Taking a higher dose of Pralex than recommended
If you have taken more Pralex than prescribed, you should contact your doctor or go to the emergency room immediately. Do this even if you do not feel any discomfort. Symptoms of overdose include dizziness, tremors, agitation, convulsions, coma, nausea, vomiting, heart rhythm disorders, low blood pressure, and electrolyte disturbances. Take the Pralex packaging with you to the doctor or hospital.
Missing a dose of Pralex
Do not take a double dose to make up for a forgotten dose. If you forget to take a dose and remember before bedtime, you should take the missed dose immediately. Take the next dose at the usual time the next day. If you remember that you have missed a dose in the night or the next day, you should skip the missed dose and take the next dose as usual.
Stopping treatment with Pralex
Do not stop taking Pralex unless your doctor tells you to. When you stop treatment, it is usually recommended to gradually reduce the dose of Pralex over a period of several weeks.
After stopping treatment with Pralex, especially if it is stopped abruptly, you may experience withdrawal symptoms. These symptoms are common when treatment with Pralex is stopped. The risk is higher if Pralex has been taken for a long time, in high doses, or if the dose has been reduced too quickly. In most patients, the symptoms are mild and disappear on their own within two weeks. In some patients, however, they may be more severe or last longer (2-3 months or longer). If you experience severe withdrawal symptoms after stopping treatment with Pralex, you should contact your doctor. Your doctor may recommend that you start taking the medicine again and taper it off more slowly.
Withdrawal symptoms include dizziness (unsteady gait, balance disorders), tingling sensation, burning sensation, and (less commonly) a sensation like an electric shock, also in the head, sleep disturbances (vivid dreams, nightmares, insomnia), restlessness, headache, nausea (feeling sick), sweating (including night sweats), agitation or excitement, tremors, disorientation, emotional instability or irritability, diarrhea (loose stools), vision disturbances, heart palpitations or irregular heartbeat.
If you have any doubts about taking this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Pralex can cause side effects, although not everybody gets them.
Side effects are usually mild and disappear after a few weeks of treatment. Remember that some of these side effects may also be symptoms of your illness and will improve as you get better.
If you experience any of the following side effects, you should contact your doctor or go to the hospital immediately:
Uncommon (may affect up to 1 in 100 people):
- unusual bleeding, including gastrointestinal bleeding
Rare (may affect up to 1 in 1,000 people):
- swelling of the skin, tongue, lips, throat, or face, hives, or difficulty breathing or swallowing (severe allergic reaction)
- high fever, agitation, confusion (disorientation), tremors, and sudden muscle contractions. These may be symptoms of a rare disorder called serotonin syndrome.
Unknown (frequency cannot be estimated from the available data):
- difficulty urinating
- seizures, see also section "Warnings and precautions"
- yellowing of the skin and whites of the eyes, which may be a sign of liver problems and/or hepatitis
- fast or irregular heartbeat, fainting, which may be symptoms of life-threatening heart rhythm disorders called torsades de pointes
- suicidal thoughts and behaviors, see also section "Warnings and precautions"
- sudden swelling of the skin or mucous membranes (angioedema)
In addition to the above, the following side effects have been reported with medicines of the same class as escitalopram (the active substance of Pralex):
- psychomotor restlessness (akathisia)
- loss of appetite
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. You can also report side effects directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl
You can also report side effects to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Pralex
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label or carton. The expiry date refers to the last day of the month stated.
There are no special storage requirements.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Pralex contains
- The active substance is escitalopram. Each tablet contains 5 mg, 10 mg, 15 mg, or 20 mg of escitalopram (as oxalate).
- The other ingredients are:
- Core: microcrystalline cellulose, butylhydroxytoluene (E 321), butylhydroxyanisole (E 320), sodium croscarmellose, silicon dioxide, talc, and magnesium stearate.
- Coating: hypromellose 5 cPs, macrogol 400, and titanium dioxide (E 171).
What Pralex looks like and contents of the pack
5 mg:White or almost white, round, coated tablets, convex on both sides, marked with the letter "F" on one side and the number "53" on the other side.
10 mg:White or almost white, oval, coated tablets, convex on both sides, marked with the letter "F" on one side and the number "54" on the other side with a deep groove dividing the "5" and "4".
15 mg:White or almost white, oval, coated tablets, convex on both sides, marked with the letter "F" on one side and the number "55" on the other side with a deep groove dividing the "5" and "5".
20 mg:White or almost white, oval, coated tablets, convex on both sides, marked with the letter "F" on one side and the number "56" on the other side with a deep groove dividing the "5" and "6".
Pack sizes:
PVC/Aclar/Aluminum blister: 28, 56, and 90 coated tablets.
Not all pack sizes may be marketed.
Marketing authorization holder
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
Manufacturer
Orion Corporation, Orion Pharma
Orionintie 1
FI-02200 Espoo
Finland
APL Swift Services (Malta) Ltd.
HF26, Hal Far Industrial Estate
Hal Far, Birzebbugia BBG 3000
Malta
To obtain more detailed information on this medicine, please contact the local representative of the marketing authorization holder:
Orion Pharma Poland Sp. z o. o.
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Country | Medicinal product name |
| Estonia | Escitalopram Orion 5 mg, 10 mg, 15 mg, 20 mg film-coated tablets |
| Finland | Escitalopram Orion 5 mg, 10 mg, 15 mg, 20 mg kalvopäällysteiset tabletit Escitalopram Orion 5 mg, 10 mg, 15 mg, 20 mg filmdragerade tabletter |
| Latvia | Escitalopram Orion 5 mg, 10 mg, 15 mg, 20 mg apvalkotās tabletes |
| Lithuania | Escitalopram Orion 5 mg, 10 mg, 15 mg, 20 mg plėvele dengtos tabletės |
| Poland | Pralex, 5 mg, 10 mg, 15 mg, 20 mg coated tablets |
Date of last revision of the leaflet:10.01.2025
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAPL Swift Services (Malta) Ltd. Orion Corporation
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PralexDosage form: Tablets, 10 mgActive substance: escitalopramManufacturer: Biofarm Sp. z o.o.Prescription requiredDosage form: Tablets, 10 mgActive substance: escitalopramPrescription requiredDosage form: Tablets, 20 mgActive substance: escitalopramPrescription required
Alternatives to Pralex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Pralex in Spain
Alternative to Pralex in Ukraine
Online doctors for Pralex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pralex – subject to medical assessment and local rules.









