
How to use Pomalidomide Glenmark
Leaflet accompanying the packaging: patient information
Pomalidomide Glenmark, 1 mg, hard capsules
Pomalidomide Glenmark, 2 mg, hard capsules
Pomalidomide Glenmark, 3 mg, hard capsules
Pomalidomide Glenmark, 4 mg, hard capsules
Pomalidomide
It is expected that Pomalidomide Glenmark will cause severe birth defects and may lead to fetal death.
- Pomalidomide Glenmark must not be taken if the patient is pregnant or may become pregnant.
- The patient must follow the advice on contraception given in this leaflet.
The patient should carefully read the leaflet before taking Pomalidomide Glenmark, as it contains important information for them.
- The patient should keep this leaflet, so they can read it again if they need to.
- If the patient has any further questions, they should ask their doctor, pharmacist, or nurse.
- This medicine has been prescribed for a specific person. It must not be given to others. The medicine may harm them, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Pomalidomide Glenmark and what is it used for
- 2. Important information before taking Pomalidomide Glenmark
- 3. How to take Pomalidomide Glenmark
- 4. Possible side effects
- 5. How to store Pomalidomide Glenmark
- 6. Contents of the pack and other information
1. What is Pomalidomide Glenmark and what is it used for
What is Pomalidomide Glenmark
Pomalidomide Glenmark contains the active substance pomalidomide. This medicine is similar to thalidomide and belongs to a group of medicines that affect the body's immune system (natural defense system).
What is Pomalidomide Glenmark used for
Pomalidomide Glenmark is used to treat adults with a type of cancer called multiple myeloma.
Pomalidomide Glenmark is used in combination with:
- two other medicines- bortezomib (a type of chemotherapy medicine) and dexamethasone (an anti-inflammatory medicine) in patients who have already received at least one other type of treatment that includes lenalidomide. or
- one other medicine- dexamethasone in patients with multiple myeloma that has worsened despite receiving at least two other types of treatment that include lenalidomide and bortezomib.
What is multiple myeloma
Multiple myeloma is a type of cancer that affects a certain type of white blood cell called plasma cells. These cells grow out of control and accumulate in the bone marrow, causing damage to bones and kidneys.
Multiple myeloma is generally incurable. However, treatment can help relieve symptoms and make them disappear for a while. This is called a "response" to treatment.
How does Pomalidomide Glenmark work
Pomalidomide Glenmark works in several ways:
- by stopping the growth of myeloma cells,
- by stimulating the immune system to attack cancer cells,
- by preventing the formation of blood vessels that supply cancer cells.
Benefits of taking Pomalidomide Glenmark with bortezomib and dexamethasone
Pomalidomide Glenmark, when taken with bortezomib and dexamethasone in patients who have already received at least one other type of treatment, may slow down the progression of multiple myeloma:
- Pomalidomide Glenmark taken with bortezomib and dexamethasone usually delayed the return of multiple myeloma by 11 months - compared to 7 months in patients who took only bortezomib and dexamethasone.
Benefits of taking Pomalidomide Glenmark with dexamethasone
Pomalidomide Glenmark, when taken with dexamethasone in patients who have already received at least two other types of treatment, may slow down the progression of multiple myeloma.
- Pomalidomide Glenmark taken with dexamethasone usually delayed the return of multiple myeloma by 4 months - compared to 2 months in patients who took only dexamethasone.
2. Important information before taking Pomalidomide Glenmark
When not to take Pomalidomide Glenmark
- if the patient is pregnant, thinks they may be pregnant, or plans to have a baby, as Pomalidomide Glenmark is expected to be harmful to the fetus (men and women taking this medicine must read the section "Pregnancy, contraception, and breastfeeding - information for men and women" below).
- if the patient may become pregnant, unless they use all necessary methods to prevent pregnancy (see the section "Pregnancy, contraception, and breastfeeding - information for men and women"). If the patient may become pregnant, the doctor will ensure that the patient understands all necessary actions to prevent pregnancy and will provide confirmation to the patient.
- if the patient is allergic to pomalidomide or any of the other ingredients of this medicine (listed in section 6). If the patient thinks they may be allergic, they should ask their doctor for advice. If the patient is not sure whether any of the above applies to them, they should ask their doctor, pharmacist, or nurse before taking Pomalidomide Glenmark.
Warnings and precautions
Before taking Pomalidomide Glenmark, the patient should discuss with their doctor, pharmacist, or nurse if:
- they have ever had blood clots in the past. During treatment with Pomalidomide Glenmark, there is an increased risk of blood clots in veins and arteries. The doctor may prescribe additional treatment (e.g., warfarin) or reduce the dose of Pomalidomide Glenmark to reduce the risk of blood clots.
- they have ever had an allergic reaction, such as a rash, itching, swelling, dizziness, or difficulty breathing while taking similar medicines called "thalidomide" and "lenalidomide".
- they have had a heart attack, have heart failure, have breathing difficulties, or smoke, have high blood pressure, or high cholesterol levels.
- they have extensive cancer spread in their body, including in the bone marrow. This can lead to a condition where tumors break down and abnormal levels of chemicals occur, which can lead to kidney failure. The patient may also experience irregular heartbeats. This condition is called tumor lysis syndrome.
- they have or have had nerve damage (causing numbness or pain in the hands or feet).
- they have or have had a hepatitis B virus infection. Taking Pomalidomide Glenmark may cause the virus to reactivate in previously infected patients, leading to a return of the infection. The doctor should check if the patient has been infected with hepatitis B in the past.
- they have or have had any of the following symptoms: rash on the face or widespread rash, redness of the skin, high fever, flu-like symptoms, swollen lymph nodes (severe skin reactions called "Drug Reaction with Eosinophilia and Systemic Symptoms" (DRESS) or "Hypersensitivity Reaction", Toxic Epidermal Necrolysis (TEN), or Stevens-Johnson Syndrome (SJS)). See also section 4 "Possible side effects".
The patient should remember that in patients with multiple myeloma treated with pomalidomide, additional types of cancer may occur. Therefore, the treating doctor should carefully evaluate the benefits and risks of prescribing this medicine to the patient.
At any time during or after treatment, the patient should immediately inform their doctor or nurse if they experience: vision disturbances, loss of vision, or double vision, difficulty speaking, weakness in the arms or legs, changes in walking or balance problems, persistent numbness, reduced sensation, or loss of sensation, memory loss, or disorientation. These symptoms may indicate a serious and potentially life-threatening brain disease called progressive multifocal leukoencephalopathy (PML). If these symptoms occurred before taking Pomalidomide Glenmark, the patient should inform their doctor about any changes in these symptoms.
After completing treatment, all unused capsules should be returned to a pharmacy that accepts them.
Pregnancy, contraception, and breastfeeding - information for men and women
As stated, during treatment with Pomalidomide Glenmark, pregnancy prevention measures must be followed.
Women taking Pomalidomide Glenmark must not become pregnant, and partners of men taking Pomalidomide Glenmark must not become pregnant, as this medicine is expected to be harmful to the fetus. The patient and their partner should use effective contraception during treatment with this medicine.
Women
Pomalidomide Glenmark must not be taken if the patient is pregnant, thinks they may be pregnant, or plans to have a baby, as this medicine is expected to be harmful to the fetus. Before starting treatment, the patient should tell their doctor if they may become pregnant, even if they think it is unlikely.
If the patient may become pregnant:
- they must use effective methods of contraception for at least 4 weeks before starting treatment, during treatment, and for at least 4 weeks after completing treatment. The patient should discuss with their doctor which method of contraception is best for them.
- each time the medicine is prescribed, the doctor will ensure that the patient understands all necessary actions to prevent pregnancy.
- the doctor will order pregnancy tests before treatment, at least every 4 weeks during treatment, and at least 4 weeks after completing treatment.
If the patient becomes pregnant despite using preventive measures:
- they must stop treatment immediately and inform their doctor immediately.
Breastfeeding
It is not known whether Pomalidomide Glenmark passes into human milk. If the patient is breastfeeding or plans to breastfeed, they should tell their doctor. The doctor will advise whether to stop or continue breastfeeding.
Men
Pomalidomide Glenmark passes into human semen.
- If the patient's partner is pregnant or may become pregnant, the patient must use condoms during the entire treatment period and for 7 days after completing treatment.
- If the patient's partner becomes pregnant while the patient is taking Pomalidomide Glenmark, the patient should immediately inform their doctor. The partner should also immediately consult a doctor.
The patient should not donate semen during treatment and for 7 days after completing treatment.
Blood donation and blood tests
During treatment and for 7 days after completing treatment, the patient should not donate blood.
Before and during treatment with Pomalidomide Glenmark, the doctor will regularly order blood tests for the patient. This is necessary because this medicine may cause a decrease in the number of blood cells (white blood cells) that help fight infections and a decrease in the number of cells (platelets) that help stop bleeding.
The doctor will order blood tests:
- before treatment,
- weekly during the first 8 weeks of treatment,
- then at least once a month for as long as the patient takes Pomalidomide Glenmark.
The doctor may change the dose of Pomalidomide Glenmark or stop treatment based on the patient's blood test results. The doctor may also change the dose or stop treatment due to the patient's overall health.
Children and adolescents
Pomalidomide Glenmark is not recommended for use in children and adolescents under 18 years of age.
Pomalidomide Glenmark and other medicines
The patient should tell their doctor, pharmacist, or nurse about all medicines they are taking or have recently taken, as well as any medicines they plan to take, as Pomalidomide Glenmark may affect the action of other medicines. Other medicines may also affect the action of Pomalidomide Glenmark.
Before taking Pomalidomide Glenmark, the patient should inform their doctor, pharmacist, or nurse, in particular, if they are taking any of the following medicines:
- certain antifungal medicines, such as ketoconazole
- certain antibiotics (e.g., ciprofloxacin, enoxacin)
- certain antidepressant medicines, such as fluvoxamine.
Driving and using machines
While taking Pomalidomide Glenmark, some people may feel tired, dizzy, faint, confused, or have reduced alertness. If these symptoms occur, the patient should not drive, use tools, or operate machinery.
Pomalidomide Glenmark contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per capsule, which is essentially "sodium-free".
3. How to take Pomalidomide Glenmark
Pomalidomide Glenmark must be prescribed by a doctor who has experience in treating multiple myeloma.
This medicine should always be taken exactly as the doctor has instructed. If the patient is unsure, they should ask their doctor, pharmacist, or nurse.
When to take Pomalidomide Glenmark with other medicines
Pomalidomide Glenmark with bortezomib and dexamethasone
- The patient should read the leaflets that come with bortezomib and dexamethasone for more information on their use and effects.
- Pomalidomide Glenmark, bortezomib, and dexamethasone are taken in treatment cycles. Each cycle lasts 21 days (3 weeks).
- The patient should look at the table below to see which medicines to take on which days of the 3-week cycle:
- each day, check the table to see which medicines to take on that day.
- on some days, the patient will take all three medicines, on some days two medicines, on some days one medicine, and on some days no medicines.
POM:Pomalidomide Glenmark; BOR: bortezomib; DEX: dexamethasone
Cycles 1 to 8 Cycles 9 and onwards
Medicine name Medicine name
Day POM BOR DEX Day POM BOR DEX
1
√
√
√
1
√
√
√
2
√
√
2
√
√
3
√
3
√
4
√
√
√
4
√
5
√
√
5
√
6
√
6
√
7
√
7
√
8
√
√
√
8
√
√
√
9
√
√
9
√
√
10
√
10
√
11
√
√
√
11
√
12
√
√
12
√
13
√
13
√
14
√
14
√
15
15
16
16
17
17
18
18
19
19
20
20
21
21
- After completing each 3-week cycle, the patient should start a new cycle.
Pomalidomide Glenmark only with dexamethasone
- The patient should read the leaflet that comes with dexamethasone for more information on its use and effects.
- Pomalidomide Glenmark and dexamethasone are taken in treatment cycles. Each cycle lasts 28 days (4 weeks).
- The patient should look at the table below to see which medicines to take on which days of the 4-week cycle:
- each day, check the table to see which medicines to take on that day.
- on some days, the patient will take both medicines, on some days one medicine, and on some days no medicines.
POM:Pomalidomide Glenmark; DEX: dexamethasone
Medicine name
Day POM DEX
1
√
√
2
√
3
√
4
√
5
√
6
√
7
√
8
√
√
9
√
10
√
11
√
12
√
13
√
14
√
15
√
√
16
√
17
√
18
√
19
√
20
√
21
√
22
√
23
24
25
26
27
28
- After completing each 4-week cycle, the patient should start a new cycle.
What dose of Pomalidomide Glenmark to take with other medicines
Pomalidomide Glenmark with bortezomib and dexamethasone
- The recommended starting dose of Pomalidomide Glenmark is 4 mg per day.
- The recommended starting dose of bortezomib will be determined by the doctor based on the patient's weight and body surface area (1.3 mg/m2 body surface area).
- The recommended starting dose of dexamethasone is 20 mg per day. However, if the patient is over 75 years old, the recommended starting dose is 10 mg per day.
Pomalidomide Glenmark only with dexamethasone
- The recommended dose of Pomalidomide Glenmark is 4 mg per day.
- The recommended starting dose of dexamethasone is 40 mg per day. However, if the patient is over 75 years old, the recommended starting dose is 20 mg per day.
The doctor may reduce the dose of Pomalidomide Glenmark, bortezomib, or dexamethasone, or stop treatment with one or more of these medicines based on the patient's blood test results, their overall health, the use of other medicines (e.g., ciprofloxacin, enoxacin, and fluvoxamine), and if the patient experiences side effects due to treatment (especially rash or swelling).
If the patient has kidney or liver disease, the doctor will closely monitor their health while taking this medicine.
How to take Pomalidomide Glenmark
- Do not break, open, or chew the capsules. If the powder from a damaged capsule comes into contact with the skin, wash the skin immediately with soap and water.
- Healthcare professionals, caregivers, and family members should wear disposable gloves when handling the blister or capsule. The gloves should then be carefully removed to avoid skin exposure, placed in a sealed plastic bag, and disposed of according to local regulations. Then, wash hands thoroughly with soap and water. Pregnant women or those who think they may be pregnant should not handle the blister or capsule.
- Swallow the capsules whole with a glass of water.
- The capsules can be taken with or without food.
- Take the capsules at about the same time each day.
To remove a capsule from the blister, press the capsule only on one side and push it through the foil. Do not press the center of the capsule, as this may damage it.
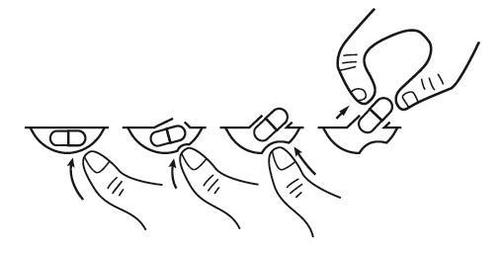
If the patient has kidney disease and is on dialysis, the doctor will advise on how and when to take Pomalidomide Glenmark.
Duration of treatment with Pomalidomide Glenmark
The patient should continue treatment cycles until the doctor decides to stop treatment.
Taking more than the recommended dose of Pomalidomide Glenmark
If the patient takes more Pomalidomide Glenmark than they should, they should immediately inform their doctor or go to the hospital. They should take the medicine packaging with them.
Missing a dose of Pomalidomide Glenmark
If the patient misses a dose of Pomalidomide Glenmark on the day they should take it, they should take the next capsule according to the scheduled dosing regimen. They should not take a double dose to make up for the missed dose.
If the patient has any further questions about taking this medicine, they should ask their doctor or pharmacist.
4. Possible side effects
Like all medicines, Pomalidomide Glenmark can cause side effects, although not everybody gets them.
Severe side effects
If the patient experiences any of the following severe side effects, they should stop taking Pomalidomide Glenmark and immediately contact their doctor – they may need urgent medical treatment.
- fever, chills, sore throat, cough, mouth sores, or any other signs of infection (due to a lower number of white blood cells that help fight infections)
- unexplained bleeding or bruising, including nosebleeds, bleeding from the gut or stomach (due to the effect of the medicine on blood cells called platelets)
- rapid breathing, rapid heartbeat, fever, and chills, passing very little or no urine, nausea, and vomiting, confusion, loss of consciousness (due to a blood infection called sepsis or septic shock)
- severe, persistent, or bloody diarrhea (also with stomach pain or fever) caused by bacteria called Clostridium difficile
- chest pain or leg pain and swelling, especially in the legs and ankles (caused by blood clots)
- shortness of breath (due to severe chest infections, pneumonia, heart failure, or blood clots)
- swelling of the face, lips, tongue, and throat, which can cause difficulty breathing (due to serious allergic reactions called angioedema and anaphylaxis)
- certain types of skin cancer (squamous cell carcinoma and basal cell carcinoma), which can cause changes in the appearance of the skin or the development of skin growths. If the patient notices any changes in their skin while taking Pomalidomide Glenmark, they should inform their doctor as soon as possible.
- reactivation of hepatitis B virus, which can cause yellowing of the skin and whites of the eyes (jaundice), dark urine, pale stools, stomach pain, fever, nausea, and vomiting. If the patient notices any of these symptoms, they should immediately contact their doctor.
- widespread rash, high body temperature, swollen lymph nodes, and involvement of other organs (drug reaction with eosinophilia and systemic symptoms, also known as DRESS or hypersensitivity reaction, toxic epidermal necrolysis, or Stevens-Johnson syndrome). If these symptoms occur, the patient should immediately stop taking pomalidomide and contact their doctor or seek immediate medical attention. See also section 2.
If the patient experiences any of the above severe side effects, they should stop taking Pomalidomide Glenmark and immediately contact their doctor– they may need urgent medical treatment.
Other side effects
Very common(may affect more than 1 in 10 people):
- shortness of breath (dyspnea)
- lung infections (pneumonia and bronchitis)
- infections of the nose, sinuses, and throat caused by bacteria or viruses
- flu-like symptoms (influenza)
- decrease in red blood cells, which can cause anemia leading to tiredness and weakness
- low levels of potassium in the blood (hypokalemia), which can cause weakness, muscle cramps, muscle pain, irregular heartbeat, tingling, or numbness, shortness of breath, mood changes
- high blood sugar levels
- rapid and irregular heartbeat (atrial fibrillation)
- loss of appetite
- constipation, diarrhea, or nausea
- vomiting
- stomach pain
- lack of energy
- difficulty sleeping or maintaining sleep
- dizziness, tremors
- muscle cramps, muscle weakness
- bone pain, back pain
- numbness, tingling, or burning sensation in the skin, pain in the hands or feet (peripheral sensory neuropathy)
- swelling of the body, including hands and feet
- urinary tract infections, which can cause burning during urination or frequent urination
Common(may affect up to 1 in 10 people):
- falls
- bleeding in the brain
- decreased ability to move or feel in the hands, arms, feet, and legs due to nerve damage (peripheral sensory-motor neuropathy)
- numbness, itching, and tingling of the skin (paresthesia)
- dizziness that makes it difficult to maintain posture and walk
- swelling caused by fluid accumulation
- hives
- itching of the skin
- shingles
- heart attack (chest pain radiating to the arms, neck, jaw, sweating, or shortness of breath, nausea, or vomiting)
- chest pain, infection in the chest
- high blood pressure
- low blood pressure, which can cause dizziness or fainting
- decrease in the number of blood cells (red and white blood cells and platelets), which can cause increased susceptibility to bleeding and bruising. The patient may feel tired, weak, and short of breath, and be more prone to infections.
- decrease in the number of lymphocytes (a type of white blood cell), often caused by infection (lymphopenia)
- low levels of magnesium in the blood (hypomagnesemia), which can cause fatigue, general weakness, muscle cramps, irritability, and low levels of calcium in the blood (hypocalcemia), which can cause numbness and tingling of the hands, feet, or lips, muscle cramps, muscle weakness, dizziness, or confusion
- low levels of phosphate in the blood (hypophosphatemia), which can cause muscle weakness and irritability or confusion
- high levels of calcium in the blood (hypercalcemia), which can cause slow reflexes and muscle weakness
- high levels of potassium in the blood, which can cause irregular heartbeat
- low levels of sodium in the blood, which can cause fatigue and confusion, tremors, seizures (convulsions), or coma
- high levels of uric acid in the blood, which can lead to gout
- low blood pressure, which can cause dizziness or fainting
- mouth pain or dry mouth
- changes in taste
- bloating
- confusion
- depression (depression)
- loss of consciousness, fainting
- cloudy vision (cataract)
- kidney damage
- inability to urinate
- abnormal liver test results
- pelvic pain
- weight loss
Uncommon(may affect up to 1 in 100 people):
- stroke
- liver inflammation, which can cause itching of the skin, yellowing of the skin and whites of the eyes (jaundice), pale stools, dark urine, stomach pain
- breakdown of cancer cells, leading to the release of toxic substances into the blood (tumor lysis syndrome), which can cause kidney problems.
- underactive thyroid gland, which can cause symptoms such as fatigue, lethargy, muscle weakness, slow heart rate, weight gain
Frequency not known(cannot be estimated from the available data):
- rejection of a transplanted organ (such as a heart or liver)
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw
tel.: +48 22 49 21 301, fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, the patient can help provide more information on the safety of this medicine.
5. How to store Pomalidomide Glenmark
The medicine should be stored out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the blister and carton after EXP. The expiry date refers to the last day of the month stated.
There are no special storage instructions for this medicine.
Do not use Pomalidomide Glenmark if the patient notices any damage or signs of opening of the packaging.
Medicines should not be disposed of via wastewater or household waste. After completing treatment, any unused capsules should be returned to a pharmacy that accepts them. This will help protect the environment.
6. Contents of the pack and other information
What Pomalidomide Glenmark contains
- The active substance is pomalidomide.
- The other ingredients are microcrystalline cellulose, maltodextrin, and sodium stearyl fumarate.
Pomalidomide Glenmark 1 mg hard capsules:
- Each capsule contains 1 mg of pomalidomide.
- The capsule shell contains: gelatin, titanium dioxide (E 171), yellow iron oxide (E 172), red iron oxide (E 172), and white ink.
- The printing ink contains: shellac, titanium dioxide (E 171), propylene glycol.
Pomalidomide Glenmark 2 mg hard capsules:
- Each capsule contains 2 mg of pomalidomide.
- The capsule shell contains: gelatin, titanium dioxide (E 171), yellow iron oxide (E 172), red iron oxide (E 172), and white ink.
- The printing ink contains: shellac, titanium dioxide (E 171), propylene glycol.
Pomalidomide Glenmark 3 mg hard capsules:
- Each capsule contains 3 mg of pomalidomide.
- The capsule shell contains: gelatin, titanium dioxide (E 171), yellow iron oxide (E 172), red iron oxide (E 172), indigo carmine (E 132), and white ink.
- The printing ink contains: shellac, titanium dioxide (E 171), propylene glycol.
Pomalidomide Glenmark 4 mg hard capsules:
- Each capsule contains 4 mg of pomalidomide.
- The capsule shell contains: gelatin, titanium dioxide (E 171), yellow iron oxide (E 172), red iron oxide (E 172), indigo carmine (E 132), erythrosine (E 127), and white ink.
- The printing ink contains: shellac, titanium dioxide (E 171), propylene glycol.
What Pomalidomide Glenmark looks like and contents of the pack
Pomalidomide Glenmark 1 mg hard capsules (capsules): yellow and red, hard gelatin capsules with "PLM 1" printed in white along the axis of the capsule body.
Pomalidomide Glenmark 2 mg hard capsules (capsules): orange and red, hard gelatin capsules with "PLM 2" printed in white along the axis of the capsule body.
Pomalidomide Glenmark 3 mg hard capsules (capsules): turquoise and red, hard gelatin capsules with "PLM 3" printed in white along the axis of the capsule body.
Pomalidomide Glenmark 4 mg hard capsules (capsules): dark blue and red, hard gelatin capsules with "PLM 4" printed in white along the axis of the capsule body.
Available pack sizes:
Each pack contains 14, 21, 14x1, or 21x1 capsules in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder
Glenmark Pharmaceuticals s.r.o.
Hvězdova 1716/2b
140 78 Prague 4
Czech Republic
Manufacturer
Glenmark Pharmaceuticals s.r.o.
Fibichova 143
566 17 Vysoké Mýto
Czech Republic
Synthon Hispania S.L.
Calle De Castello 1
Sant Boi De Liobregat
08830 Barcelona
Spain
Synthon B.V.
Microweg 22
Nijmegen
6545 CM Gelderland
Netherlands
This medicine is authorized in the Member States of the European Economic Area under the following names:
| Member State | Marketing authorization name |
| Netherlands | Pomalidomide Glenmark 1 mg hard capsules Pomalidomide Glenmark 2 mg hard capsules Pomalidomide Glenmark 3 mg hard capsules Pomalidomide Glenmark 4 mg hard capsules |
| Denmark | Pomalidomide Glenmark |
| Finland | Pomalidomide Glenmark |
| Italy | Pomalidomide Glenmark 1 mg capsule rigide Pomalidomide Glenmark 2 mg capsule rigide Pomalidomide Glenmark 3 mg capsule rigide Pomalidomide Glenmark 4 mg capsule rigide |
| Norway | Pomalidomide Glenmark |
| Poland | Pomalidomide Glenmark |
| Sweden | Pomalidomide Glenmark |
For more information about this medicine, the patient should contact their local representative of the marketing authorization holder:
Glenmark Pharmaceuticals Sp. z o. o.
ul. Dziekońskiego 3
00-728 Warsaw
Email: [email protected]
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGlenmark Pharmaceuticals s.r.o. Synthon B.V. Synthon Hispania S.L.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Pomalidomide GlenmarkDosage form: Capsules, 3 mgActive substance: pomalidomidePrescription requiredDosage form: Capsules, 4 mgActive substance: pomalidomidePrescription requiredDosage form: Capsules, 2 mgActive substance: pomalidomidePrescription required
Alternatives to Pomalidomide Glenmark in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Pomalidomide Glenmark in Spain
Alternative to Pomalidomide Glenmark in Ukraine
Online doctors for Pomalidomide Glenmark
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pomalidomide Glenmark – subject to medical assessment and local rules.






