

Pliaglis

Ask a doctor about a prescription for Pliaglis

How to use Pliaglis
Package Leaflet: Information for the Patient
Pliaglis, 70 mg/g + 70 mg/g, Cream
Lidocaine + Tetracaine
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or nurse. See section 4.
Contents of the Package Leaflet:
- 1. What is Pliaglis and what is it used for
- 2. Important information before using Pliaglis
- 3. How to use Pliaglis
- 4. Possible side effects
- 5. How to store Pliaglis
- 6. Contents of the pack and other information
1 What is Pliaglis and what is it used for
Pliaglis is a cream containing the local anaesthetics lidocaine and tetracaine, which are used to numb the skin area before a painful procedure, such as needle insertion or laser treatment.
2 Important information before using Pliaglis
When not to use Pliaglis
if you are allergic to lidocaine or tetracaine, other similar local anaesthetics or any of the other ingredients of this medicine (listed in section 6) if you are allergic to para-aminobenzoic acid (also known as PABA), which is formed by the breakdown of tetracaine in the body, methyl parahydroxybenzoate (E 218) or propyl parahydroxybenzoate (E 216) on damaged or irritated skin on mucous membranes such as the mouth
Warnings and precautions
Before starting treatment with Pliaglis, discuss it with your doctor or pharmacist:
- if you have liver, kidney or heart problems
- if you have an acute illness or are physically weakened, as these conditions may increase your sensitivity to Pliaglis Avoid contact with the eyes. If Pliaglis gets into your eyes, rinse immediately with water or saline solution and protect them until sensation returns. Do not use Pliaglis for longer than recommended. See section 3. After removing Pliaglis, a feeling of numbness of the skin will occur. Avoid scratching or rubbing the numb area or touching very hot or very cold surfaces until the numbness has resolved, as you may accidentally injure your skin.
Children and adolescents
Do not give Pliaglis to children and adolescents under 18 years of age, as its safety and efficacy in children and adolescents have not been established.
Pliaglis and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take. The risk of side effects increases when using Pliaglis with other medicines, such as:
- certain heart medicines such as quinidine, disopyramide, tocainide, mexiletine and amiodarone
- medicines known to cause methaemoglobinaemia such as: sulphonamides, naphthalene, nitrites and nitrates, nitrofurantoin, nitroglycerin, sodium nitroprusside, primaquine and quinine
- other medicines containing lidocaine and/or tetracaine
Using Pliaglis with food and drink
Pliaglis can be used before or after food or drink.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Pregnancy
Consult your doctor before using Pliaglis during pregnancy. Breastfeeding
You can breastfeed while using Pliaglis, provided that the medicine is not applied to the breast.
Driving and using machines
Pliaglis has no or negligible influence on the ability to drive and use machines.
Pliaglis contains methyl parahydroxybenzoate (E 218) and propyl parahydroxybenzoate (E 216)
These substances may cause allergic reactions (possibly delayed).
3 How to use Pliaglis
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist. The recommended dose is approximately 1.3 g of cream per 10 cm².
Pliaglis is intended for use on a single patient.
Pliaglis should only be applied to dry, intact skin.
DO NOT apply Pliaglis to the face yourself.
Only a doctor should apply Pliaglis to the face.
Pliaglis should be spread evenly and thinly (about 1 mm thick) onto the area to be treated (as determined by your doctor), using a flat tool such as a metal spatula or tongue depressor. Never apply Pliaglis with your fingers.
The area where Pliaglis is applied should not be covered with an occlusive dressing.
Do not touch the cream with your fingers.
Avoid contact with the eyes. If Pliaglis gets into your eyes, rinse immediately with water or saline solution and protect them until sensation returns.
Leave the cream to dry for 30 to 60 minutes, depending on the procedure, as advised by your doctor.
After the required application time, the dried cream will form a soft mask on the skin. Pliaglis can be removed by grasping the edge of the mask and peeling it off the skin.
The mask should be carefully removed immediately after peeling (more information on how to remove the mask can be found in section 5).
Any remaining mask on the skin surface should be wiped off with a cotton ball (or gauze).
Wash your hands immediately after peeling and removing the mask.
Using more Pliaglis than recommended
The maximum application area should not exceed 400 cm² (do not use more than two 30 g tubes). If you use too much Pliaglis, consult your doctor or pharmacist immediately.
If you think you need more Pliaglis than recommended,consult your doctor immediately.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4 Possible side effects
Like all medicines, Pliaglis can cause side effects, although not everybody gets them. Most side effects occur at the site where the cream is applied to the skin. They are usually mild, short-lived and resolve after treatment is stopped.
The two active substances in Pliaglis (lidocaine and tetracaine) may cause allergic reactions (anaphylactoid reactions) such as rash, swelling and difficulty breathing. If you experience any of these side effects, remove Pliaglis and contact your doctor immediately.
Pliaglis and contact your doctor.
Most side effects occur at the application site.
Very common: may affect more than 1 in 10 people
redness of the skin
discolouration of the skin
Common: may affect up to 1 in 10 people
swelling of the skin
Uncommon side effects: may affect up to 1 in 100 people
itching of the skin
pain or tenderness of the skin
Rare: may affect up to 1 in 1,000 people
pallor of the skin
burning sensation of the skin
swelling of the face
desquamation of the skin
irritation of the skin
tingling of the skin
swelling of the eyelid
Unknown: frequency cannot be estimated from the available data
urticaria
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Tel.: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorisation holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5 How to store Pliaglis
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the tube and carton.
The expiry date refers to the last day of that month.
Store in a refrigerator (2°C – 8°C), including after opening.
Do not freeze.
After opening, the packaging should be used within 3 months. It is recommended to write the opening date on the packaging.
Do not use Pliaglis if the packaging is damaged in any way.
The used mask should be disposed of with caution, as it contains a high concentration of the active substances. To protect the environment, do not flush the used mask down the toilet. The used mask should be placed in a closed container, such as a plastic bag.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6 Contents of the pack and other information
What Pliaglis contains
- The active substances are lidocaine and tetracaine; 1 g of cream contains 70 mg of lidocaine and 70 mg of tetracaine.
- The other ingredients are calcium hydrogen phosphate, anhydrous, purified water, polyvinyl alcohol, white petrolatum, sorbitan monopalmitate, methyl parahydroxybenzoate (E 218) and propyl parahydroxybenzoate (E 216).
What Pliaglis looks like and contents of the pack
Pliaglis is a white or almost white cream.
A 15 g or 30 g tube with a screw cap, packaged in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder:
Egis Pharmaceuticals PLC
Keresztúri út 30-38
1106 Budapest
Hungary
Manufacturer:
Industrial Farmacéutica Cantabria, S.A.
Barrio Solía, nº 30
La Concha
Villaescusa
39690 Santander
Cantabria
Spain
This medicinal product is authorised in the Member States of the European Economic Area under the following names:
Austria, Belgium, France, Greece, Spain, Netherlands, Ireland, Luxembourg, Germany, Poland, Portugal, United Kingdom, Italy: PLIAGLIS
Denmark, Norway: PLIAPEL
Date of last revision of the leaflet:08.2022
---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Pliaglis is intended for use on a single patient.
For procedures on the face, Pliaglis should be applied by healthcare professionals.
For procedures on other parts of the body, Pliaglis should be applied by healthcare professionals or by patients who have been properly instructed in the correct use of the medicine.
It is recommended that patients and healthcare professionals avoid repeated direct contact with the cream or skin treated with the cream to prevent contact dermatitis.
Pliaglis should never be applied with the fingers.
Pliaglis should be applied using a flat tool such as a spatula or tongue depressor.
Wash your hands immediately after peeling and removing the mask.
For dermatological procedures such as pulsed dye laser treatment, laser hair removal, non-ablative laser facial rejuvenation, wrinkle injections and vein closure, apply approximately 1.3 g of Pliaglis per 10 cm² of intact skin, spreading a 1 mm thick layer for 30 minutes.
For dermatological procedures such as laser tattoo removal and laser vein removal, apply approximately 1.3 g of Pliaglis per 10 cm² of intact skin, spreading a 1 mm thick layer for 60 minutes.
- 1) Determine the size of the area to be treated. You can use the following table to determine the amount of cream to apply to achieve a 1 mm thick layer, depending on the area.
| Treatment area (cm²) | Approximate amount of Pliaglis applied (g) | |
| 10 | 1.3 | 2 x the amount of cream that fits on the tip of the finger |
| 50 | 6.5 | Half of the contents of a 15 g tube |
| 100 | 13 | The entire contents of a 15 g tube |
| 200 | 26 | The entire contents of a 30 g tube |
| 400 | 52 | The entire contents of two 30 g tubes |
The maximum application area should not exceed 400 cm².
- 2) Pliaglis should be spread evenly and thinly (about 1 mm thick) onto the area to be treated, using a flat tool such as a metal spatula or tongue depressor. Avoid contact with the eyes of the patient and the person applying the cream.
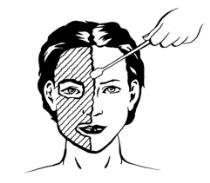
- 3) Depending on the procedure, leave the cream to dry for 30 or 60 minutes.
- 4) After the required application time, the dried cream will form an elastic mask on the skin. Pliaglis should be removed by grasping the edge of the mask and peeling it off the skin.
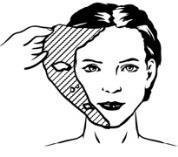
- 5) Wipe off any remaining mask from the treatment area and prepare the patient for the procedure. Skin anaesthesia may last from 2 to 13 hours after mask removal.
- 6) Immediately after peeling, dispose of the mask according to local regulations.
- 7) Wash your hands immediately after peeling and removing the mask.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterIndustrial Farmaceutica Cantabria, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PliaglisDosage form: Cream, (25 mg + 25 mg)/gActive substance: lidocaine, combinationsManufacturer: Pierre Fabre Medicament ProductionPrescription requiredDosage form: Gel, (3.3 mg + 1 mg)/gActive substance: lidocaine, combinationsManufacturer: Delpharm OrleansPrescription not requiredDosage form: Plaster, 25 mg + 25 mgActive substance: lidocaine, combinationsPrescription not required
Alternatives to Pliaglis in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Pliaglis in Spain
Alternative to Pliaglis in Ukraine
Online doctors for Pliaglis
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pliaglis – subject to medical assessment and local rules.














