
Pentazocinum Vzf
Ask a doctor about a prescription for Pentazocinum Vzf

How to use Pentazocinum Vzf
Leaflet attached to the packaging: patient information
PENTAZOCINUM WZF, 30 mg/ml, solution for injections
Pentazocinum
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Pentazocinum WZF and what is it used for
- 2. Important information before using Pentazocinum WZF
- 3. How to use Pentazocinum WZF
- 4. Possible side effects
- 5. How to store Pentazocinum WZF
- 6. Package contents and other information
1. What is Pentazocinum WZF and what is it used for
Pentazocinum WZF contains pentazocine, which has a strong analgesic effect. This medicine belongs to the group of opioid analgesics.
Pentazocinum WZF is intended for subcutaneous, intramuscular, and intravenous administration.
Pentazocinum WZF is used to combat pain of varying intensity, from moderate to severe.
2. Important information before using Pentazocinum WZF
When not to use Pentazocinum WZF:
- if the patient is allergic to pentazocine, other opioid medicines, or any of the other ingredients of this medicine (listed in section 6);
- if the patient has breathing difficulties or a disease that causes breathing difficulties or shortness of breath (called obstructive disease of the upper airways);
- if the patient has bronchial asthma, especially exacerbated asthma;
- if the patient has acute alcohol poisoning or delirium tremens;
- if the patient has suffered head injuries;
- if the patient has increased intracranial pressure (symptoms - e.g., headache, impaired consciousness and balance, vision disturbances);
- if the patient has heart failure caused by chronic lung disease.
Warnings and precautions
Before starting to use Pentazocinum WZF, the patient should discuss it with their doctor, pharmacist, or nurse.
The doctor will exercise particular caution when using pentazocine and will take appropriate action in patients:
- with reduced thyroid function (hypothyroidism);
- with reduced adrenal cortex function (too little hormone production) or so-called chromaffinoma of the adrenal gland;
- with prostate enlargement;
- with low blood pressure or cardiovascular diseases, as the medicine may cause a decrease or increase in blood pressure;
- with inflammatory bowel diseases or impaired bowel patency;
- abusing drugs;
- with biliary tract disorders - with cholecystitis (the medicine may exacerbate pain symptoms);
- with liver function disorders;
- with kidney function disorders;
- in advanced age;
- suffering from porphyria (disorders of heme production - a red pigment that is part of some enzymes);
- with pancreatitis;
- currently using or having used within the last 2 weeks antidepressant medicines from the group of monoamine oxidase inhibitors. If the patient develops dependence, sudden cessation of pentazocine administration may cause withdrawal syndrome symptoms - see section 3, subsection: "Discontinuation of Pentazocinum WZF".
Children
Pentazocinum WZF can be used in children (see section 3: "How to use Pentazocinum WZF"). In case of any doubts, consult your doctor.
Pentazocinum WZF and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Concomitant use of Pentazocinum WZF and sedative medicines, such as benzodiazepines or derivatives, increases the risk of drowsiness, breathing difficulties (respiratory depression), or coma, which can be life-threatening. Therefore, combined treatment should only be considered when other treatment methods are not available.
If Pentazocinum WZF is used together with sedative medicines, the doctor should limit the dose of the medicine and the period of concomitant use.
The patient should tell their doctor about all sedative medicines they are taking and strictly follow the prescribed dose. It may be helpful to inform a relative or close friend of the patient about the possibility of the above-mentioned symptoms. If these symptoms occur, consult a doctor.
The following medicines affect the action of pentazocine:
- monoamine oxidase inhibitors (antidepressant medicines), such as moclobemide, even if the patient has taken them within the last 2 weeks;
- tricyclic antidepressant medicines, such as amitriptyline;
- sleeping medicines, such as phenobarbital;
- sedative, anxiolytic medicines, such as diazepam;
- medicines from the phenothiazine derivative group, such as promazine, chlorpromazine;
- smoking - reduces the effectiveness of pentazocine.
Pentazocine may inhibit the action of morphine (an opioid analgesic).
Pentazocinum WZF with alcohol
Alcohol should be avoided during pentazocine treatment.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
- The use of pentazocine during pregnancy and breastfeeding will be decided by the doctor.
- Caution is recommended when using Pentazocinum WZF during premature labor. In newborns born to mothers who have taken pentazocine for a long time, withdrawal syndrome symptoms may occur - see section 3, subsection: "Discontinuation of Pentazocinum WZF".
Driving and operating machinery
Pentazocinum WZF may cause drowsiness and impair psychophysical fitness. During treatment with this medicine, the patient should not drive vehicles or operate machinery.
Pentazocinum WZF contains sodium
Pentazocinum WZF contains 0.064 mmol/ml (1.48 mg/ml) of sodium, which is less than 1 mmol (23 mg) of sodium per 1 ml, meaning the medicine is considered "sodium-free".
3. How to use Pentazocinum WZF
Pentazocinum WZF is administered by medical personnel.
- The dosage is determined individually by the doctor for each patient.
- Pentazocinum WZF is administered subcutaneously, intramuscularly, or intravenously.
- Pentazocinum WZF can be administered to children after the first year of life.
- Pentazocinum WZF should be used systematically, according to the scheme prescribed by the doctor.
- Patients who use this medicine for a long time in multiple injections per day are advised to use intramuscular injections to avoid skin hardening and tissue hardening under the skin at the injection site.
Using a higher dose of Pentazocinum WZF than recommended
Pentazocine is administered by medical personnel, so it is unlikely that the patient will receive more medicine than they should.
After using a higher dose of the medicine than recommended, the following symptoms may occur: drowsiness, deepening coma.
Breathing difficulties, significant decrease in blood pressure with heart failure may occur.
If such symptoms occur, the patient should immediately inform the medical personnel, who will take appropriate action.
Missing a dose of Pentazocinum WZF
A double dose should not be used to make up for a missed dose.
Discontinuation of Pentazocinum WZF
In case of sudden cessation of pentazocine use, especially in dependent individuals, withdrawal syndrome symptoms occur. These include: nausea, diarrhea, cough, mood disturbances, tearing, pupil dilation, runny nose, insomnia with persistent yawning, sweating, increased blood pressure, muscle tremors, "goosebumps", loss of appetite, slight increase in respiratory rate, feeling of diffuse pain in many parts of the body, very strong desire to take the medicine, and delirium. The severity of symptoms depends on the patient's condition, the size and frequency of doses, and the duration of pentazocine use. Usually, discontinuation of the medicine does not cause disturbances. In rare cases, in patients who have experienced greater difficulties, re-administration of pentazocine and then gradual reduction of doses usually alleviates the symptoms.
In case of any further doubts related to the use of this medicine, consult a doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using the medicine and immediately consult a doctor or nurse if the patient experiences the first symptoms of an allergic reaction (e.g., facial swelling, lip swelling, tongue swelling, throat swelling, causing difficulty breathing or swallowing). Such symptoms may occur after administration of
pentazocine. The doctor will decide on further action.
The following are the most common:
- nausea, vomiting;
- drowsiness;
- excessive sweating;
- dizziness;
- disorientation.
The following may also occur:
- deep changes in consciousness (confusion);
- difficulty urinating - urinary retention (rare);
- bile duct spasm (severe pain on the right side under the ribs);
- dry mouth, abdominal pain;
- skin redness, including facial skin;
- rapid heart rate, fainting, high blood pressure (rare);
- hallucinations, euphoria, disorientation, unusual dreams;
- pupil constriction, blurred vision;
- chills, tremors, seizures;
- numbness, tingling of limbs;
- constipation;
- breathing difficulties, respiratory depression.
The following have also been reported:
- headache, impaired consciousness and balance, vision disturbances (these may be symptoms of increased intracranial pressure);
- psychological and physical dependence after long-term use of pentazocine;
- withdrawal syndrome symptoms (listed in section 3, subsection: "Discontinuation of Pentazocinum WZF");
- skin inflammation, itching;
- pain and burning during injection, ulcers, nodules, skin indentation at the injection site, significant hardening of the skin and subcutaneous tissue, as well as deeper muscle tissue, muscle fibrosis;
- changes in blood composition (decrease or increase in white blood cell count);
- severe skin reactions with blistering or skin peeling and severe skin redness with swelling;
- changes in the frequency and strength of uterine contractions during labor.
Reporting side effects
If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Pentazocinum WZF
Store at a temperature below 25°C. Store the ampoules in the outer packaging to protect them from light. Do not freeze.
The medicine should be stored in a place that is out of sight and reach of children.
Do not use this medicine after the expiry date stated on the box and ampoule. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Pentazocinum WZF contains
- The active substance of the medicine is pentazocine. Each 1 ml of solution contains 30 mg of pentazocine.
- The other ingredients are: lactic acid, sodium chloride, sodium hydroxide 10% or lactic acid (to adjust pH), water for injections.
What Pentazocinum WZF looks like and what the package contains
Pentazocinum WZF is a colorless liquid.
Package: a cardboard box containing 10 ampoules of 1 ml each.
Marketing authorization holder and manufacturer
Warsaw Pharmaceutical Works Polfa S.A.
Karolkowa 22/24; 01-207 Warsaw
To obtain more detailed information, contact the local representative of the marketing authorization holder:
Warsaw Pharmaceutical Works Polfa S.A.
Karolkowa 22/24; 01-207 Warsaw
phone: 22 691 39 00
Date of the last update of the leaflet:
Information intended exclusively for healthcare professionals:
PENTAZOCINUM WZF, 30 mg/ml, solution for injections
Pentazocinum
Method of administration of Pentazocinum WZF
- Pentazocinum WZF can be administered subcutaneously, intramuscularly, or intravenously.
- If it is necessary to administer pentazocine multiple times a day for a longer period, it is better to choose intramuscular administration from the two routes of administration - intramuscular and subcutaneous.
- To reduce the risk of local tissue damage, it is recommended to systematically change the injection site.
- Pentazocinum WZF should not be mixed in the same syringe with barbiturate solutions or diazepam solutions, or infusion solutions containing sodium bicarbonate, as a precipitate may form.
Instructions for opening the ampoule
Before opening the ampoule, make sure that the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
A colored dot (see Figure 1) has been placed on each ampoule as a mark indicating the break point below it.
- To open the ampoule, hold it vertically in both hands, with the colored dot facing you - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
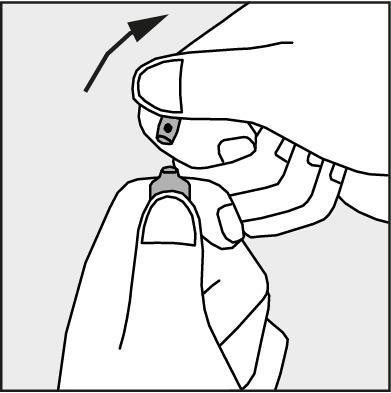
- Press in the direction of the arrow shown in Figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused product should be destroyed in accordance with applicable regulations.
Figure 1.
Figure 2.
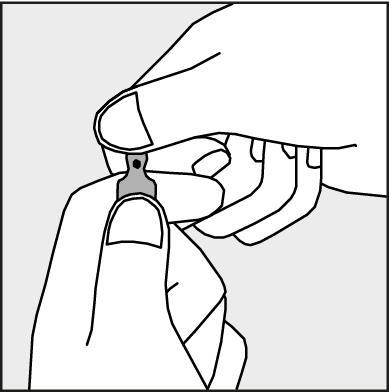
Figure 3.
Dosage
Adults and adolescents from 12 to 16 years old
Intramuscularly, subcutaneously: 45 mg to 60 mg or intravenously 30 mg every 3-4 hours. Do not exceed the dose of 60 mg intramuscularly or subcutaneously.
In the case of intravenous administration, do not exceed the dose of 30 mg of pentazocine. The maximum daily dose is 360 mg of pentazocine.
In patients with coronary heart disease, to avoid pulmonary hypertension, the dose should not exceed 150 mg per day.
Children after the first year of life to 12 years old
Parenteral administration of pentazocine in pediatrics is limited mainly to single doses in premedication before anesthesia and supplementary or postoperative pain treatment for a period not longer than 7 days.
Note
Do not exceed 1 mg/kg body weight subcutaneously or intramuscularly or 0.5 mg/kg body weight intravenously.
If multiple administration is necessary, the intervals between doses should be at least 6 hours. During pentazocine treatment, other (non-opioid) painkillers can be used.
Elderly patients
In elderly patients, who often have impaired renal or hepatic function, the doctor will recommend using smaller doses of pentazocine or increasing the interval between administered doses.
Patients with impaired renal and/or hepatic function
In patients with significant hepatic impairment, pentazocine metabolism may be impaired, and side effects may be exacerbated. Caution is also recommended in patients with impaired renal function. In both cases, the dose of pentazocine should be modified (reduced).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterWarszawskie Zakłady Farmaceutyczne POLFA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Pentazocinum Vzf
Online doctors for Pentazocinum Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pentazocinum Vzf – subject to medical assessment and local rules.














