
Pentasa

Ask a doctor about a prescription for Pentasa

How to use Pentasa
Leaflet attached to the packaging: patient information
PENTASA, 1 g/100 ml, rectal suspension
Mesalazine
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor. See section 4.
Table of contents of the leaflet:
- 1. What is Pentasa and what is it used for
- 2. Important information before using Pentasa
- 3. How to use Pentasa
- 4. Possible side effects
- 5. How to store Pentasa
- 6. Contents of the packaging and other information
1. What is Pentasa and what is it used for
Mesalazine, the active substance of Pentasa, acts locally as an anti-inflammatory agent on the diseased wall of the sigmoid colon and rectum.
Pentasa is used to treat ulcerative colitis.
2. Important information before using Pentasa
BEFORE STARTING MESALAZINE TREATMENT, THE PATIENT SHOULD INFORM THEIR DOCTOR:
- If the patient has ever experienced: severe skin rash or skin peeling, blisters or ulcers in the mouth.
When not to use Pentasa
if the patient is allergic to mesalazine or any of the other ingredients of this medicine (listed in section 6),
if the patient is allergic to salicylates, such as aspirin,
if the patient has severe liver or kidney dysfunction,
if the patient has stomach or duodenal ulcer disease,
if the patient has haemorrhagic diathesis.
Warnings and precautions
Before starting Pentasa treatment, the patient should discuss it with their doctor.
Care should be taken:
if the patient is allergic to sulfasalazine,
if there are liver or kidney function disorders,
if the patient is being treated with medicines that may affect kidney function,
if the patient is being treated with azathioprine (an immunosuppressive agent), 6-mercaptopurine (an antineoplastic and immunosuppressive agent) or tioguanine (an antineoplastic agent),
if there are lung disorders, especially bronchial asthma,
if the patient experiences severe or recurrent headache, vision disturbances or ringing or buzzing in the ears, they should contact their doctor immediately.
Mesalazine treatment may lead to the formation of kidney stones. Symptoms may include pain in the sides of the abdomen and haematuria. During mesalazine treatment, the patient should drink sufficient fluids.
Severe skin reactions, including drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported with mesalazine treatment. The patient should stop using mesalazine and seek medical attention immediately if they experience any symptoms of these severe skin reactions, listed in section 4.
Rarely, mesalazine has been reported to cause cardiac hypersensitivity reactions (myocarditis and pericarditis).
Very rarely, serious blood disorders have been reported.
If acute intolerance symptoms occur, such as colicky abdominal pain, acute abdominal pain, fever, severe headache or rash, the patient should stop treatment and consult their doctor immediately.
Usually, before and during treatment, the doctor will order blood and urine tests to assess liver, kidney or blood function.
Mesalazine may cause reddish-brown discoloration of urine after contact with sodium hypochlorite bleach in toilet water. This is a chemical reaction between mesalazine and bleach and is harmless.
Children and adolescents
Experience with the use of Pentasa in children is limited and clinical data are restricted.
Pentasa and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. In particular, this concerns medicines such as:
sulfasalazine, azathioprine, 6-mercaptopurine or tioguanine, warfarin.
Pregnancy, breastfeeding and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant or plans to have a child, they should consult their doctor before using this medicine.
Mesalazine passes through the placenta.
Mesalazine passes into breast milk.
Driving and using machines
No effect of Pentasa on the ability to drive and use machines has been observed.
3. How to use Pentasa
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor.
The recommended dose is:
Adults
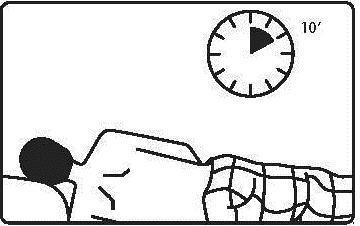
1 g (one suppository) in the evening before bedtime.
In elderly patients, there is no need to change the dosage.
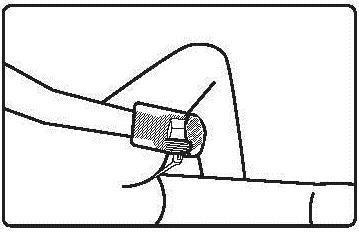
The medicine is administered rectally.
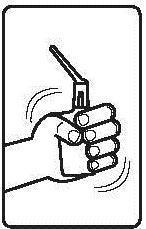
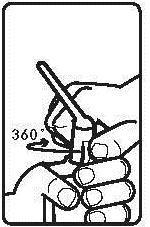
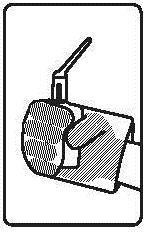
Instructions for using the medicine:
Before using the suppository, the patient should empty their bowels.
Before administration, the bottle with the suspension should be warmed to about 37°C.
|  | |
|  | |
|  | |
| ||
|  | |
|  | |
|  | |
| 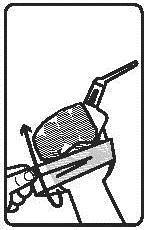 | |
The medicine should be used immediately after opening the foil pouch.
The patient should protect their clothing from staining with Pentasa, as it may cause discoloration of the material. In case of accidental staining, the material should be washed immediately.
Using a higher dose of Pentasa than recommended
In case of overdose, symptomatic treatment in a hospital and monitoring of kidney function are recommended.
4. Possible side effects
Like all medicines, Pentasa can cause side effects, although not everybody gets them.
The patient should stop using mesalazine and seek medical attention immediately if they experience any of the following symptoms:
- red, flat, patchy or circular spots on the torso, often with blisters in the centre, skin peeling, ulcers in the mouth, throat, nose, genitals and eyes, widespread rash, fever and swollen lymph nodes. The occurrence of such severe skin rashes may be preceded by fever and flu-like symptoms.
Severe side effects with unknown frequency (cannot be estimated from the available data)
- the patient should seek medical attention immediately
if the patient experiences severe or recurrent headache, vision disturbances or ringing or buzzing in the ears. These may be symptoms of increased intracranial pressure (idiopathic intracranial hypertension).
- the patient should seek medical attention immediately
The following side effectsare common(may affect 1 to 10 in 100 patients):
- diarrhoea
- nausea
- abdominal pain
- headache
- vomiting
- bloating
- rash
- hives
- discomfort in the anal area and anal irritation
- anal itching
- painful defecation
The following side effectsare rare(may affect 1 to 10 in 10,000 patients):
- dizziness
- myocarditis
- pericarditis
- increased amylase activity
- acute pancreatitis
- increased skin sensitivity to sunlight and ultraviolet radiation (photosensitivity).
The following side effectsare very rare(may affect less than 1 in 10,000 patients):
- muscle pain
- joint pain
- temporary hair loss
- allergic pneumonia, allergic reactions and fibrotic changes in the lungs (including dyspnoea, cough, bronchospasm), eosinophilic pneumonia - i.e. pulmonary infiltrate with eosinophilia, interstitial lung disease, pulmonary infiltration, pneumonia
- kidney function disorders (acute and chronic interstitial nephritis, nephrotic syndrome - i.e. a syndrome of symptoms caused by excessive loss of protein in the urine, kidney failure), urine discoloration
- increased liver enzyme activity, increased levels of cholestasis indicators (substances indicating bile stasis in the liver) and bilirubin, harmful effect on the liver (including hepatitis, cholestatic hepatitis, cirrhosis, liver failure)
- blood disorders, such as: anaemia, aplastic anaemia, agranulocytosis - i.e. a very low number of granulocytes, neutropenia - i.e. a low number of neutrophil granulocytes, leucopenia - i.e. a low number of white blood cells (including granulocytopenia - i.e. a low number of granulocytes), pancytopenia - i.e. a low number of all blood cells, thrombocytopenia - i.e. a low number of platelets and eosinophilia (as part of an allergic reaction)
- reactions similar to lupus erythematosus
- peripheral neuropathy - i.e. peripheral nerve dysfunction
- pancolitis - i.e. a severe form of ulcerative colitis, in which the disease affects the entire colon
- transient oligospermia (low sperm count in male semen)
- hypersensitivity reactions, including allergic rash, anaphylactic reaction, erythema multiforme
- drug fever.
Unknown frequency(cannot be estimated from the available data)
- kidney stones and associated kidney pain (see also section 2)
- drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS) - severe skin reactions, i.e. non-permanent blisters on mucous membranes, mainly in the mouth and genitals.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw,
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl .
Side effects can also be reported to the marketing authorisation holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Pentasa
Keep out of the sight and reach of children.
Do not store above 25°C. Store in the original packaging to protect from light.
Do not use this medicine after the expiry date stated on the packaging after the EXP label.
The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Pentasa contains
The active substance of Pentasa is mesalazine. 100 ml of suspension contains 1 g of mesalazine.
The other ingredients are: disodium edetate, sodium metabisulphite, sodium acetate, hydrochloric acid, purified water
What Pentasa looks like and contents of the packaging
Pentasa is available as a rectal suspension.
The suspension is white to slightly yellow in colour.
The packaging contains 7 bottles of 100 ml suspension
Marketing authorisation holder
Ferring GmbH
Wittland 11
D-24109 Kiel
Germany
Manufacturer
Ferring-Léčiva a.s.
K Rybniku 475
252 42 Jesenice near Prague
Czech Republic
To obtain more detailed information, the patient should contact the representative of the marketing authorisation holder.
Ferring Pharmaceuticals Poland Sp. z o.o.
Szamocka 8
01-748 Warsaw
Phone: +48 22 246 06 80, Fax: +48 22 246 06 81
Date of last revision of the leaflet:January 2025
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFerring Leciva a.s.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PentasaDosage form: Tablets, 250 mgActive substance: mesalazinePrescription requiredDosage form: Suppositories, 250 mgActive substance: mesalazineManufacturer: Temmler Italia S.R.L.Prescription requiredDosage form: Tablets, 500 mgActive substance: mesalazinePrescription required
Alternatives to Pentasa in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Pentasa in Spain
Alternative to Pentasa in Ukraine
Online doctors for Pentasa
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pentasa – subject to medical assessment and local rules.














