
Paracetamol Hasco Forte

Ask a doctor about a prescription for Paracetamol Hasco Forte

How to use Paracetamol Hasco Forte
Leaflet attached to the packaging: patient information
Paracetamol Hasco Forte
240 mg/5 ml, oral suspension
Paracetamolum
for infants and children
You should carefully read the contents of the leaflet before using the medicine, as it contains
important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by
your doctor or pharmacist.
- You should keep this leaflet, so you can read it again if you need to.
- If you need advice or additional information, you should consult a pharmacist.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
- If after 3 days there is no improvement or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Paracetamol Hasco Forte and what is it used for
- 2. Important information before using Paracetamol Hasco Forte
- 3. How to use Paracetamol Hasco Forte
- 4. Possible side effects
- 5. How to store Paracetamol Hasco Forte
- 6. Contents of the packaging and other information
1. What is Paracetamol Hasco Forte and what is it used for
Paracetamol Hasco Forte, oral suspension is an antipyretic and analgesic medicine intended for use in infants and children. When used in recommended doses, it is well tolerated.
Indications for use
Fever and pains of various origins (e.g. after surgical procedures, teething pains, mild to moderate headaches) and symptoms accompanying the body's reaction to vaccination (pain, fever, local reaction).
In addition, the medicine is used in infants from 0 to 3 months of age (with a body weight of up to 4 kg) for the symptomatic treatment of fever lasting no longer than 3 days and pain of mild to moderate severity.
If after 3 days there is no improvement or the patient feels worse, they should consult a doctor.
2. Important information before using Paracetamol Hasco Forte
When not to use Paracetamol Hasco Forte
- if the patient is hypersensitive to paracetamol or any of the other ingredients of this medicine (listed in section 6),
- in patients with severe liver failure or viral hepatitis,
- in patients with severe kidney failure,
- in alcoholism.
Warnings and precautions
Before starting to use Paracetamol Hasco Forte, you should discuss it with your doctor or pharmacist.
Do not use with other medicines containing paracetamol due to the risk of overdose. In case of overdose, consult a doctor immediately, even if the patient feels well.
Without a doctor's recommendation, do not use for more than 3 days. Do not use doses larger than recommended.
The medicine should be used with caution in patients with liver and kidney failure. There is a special risk of liver damage in malnourished individuals. Caution should be exercised when using in patients with reduced glutathione levels (such as in sepsis).
Using paracetamol may increase the risk of metabolic acidosis. Caution should be exercised when using paracetamol in patients with glucose-6-phosphate dehydrogenase deficiency and methemoglobin reductase deficiency.
During the use of Paracetamol Hasco Forte, the doctor should be informed immediately if the patient has severe diseases, including severe kidney dysfunction or sepsis (when bacteria and their toxins circulate in the blood, leading to organ damage) or malnutrition, chronic alcoholism, or if the patient is also taking flucloxacillin (an antibiotic). In these situations, patients have been reported to develop a severe disease called metabolic acidosis (a blood and body fluid disorder), when they used paracetamol in regular doses for a longer period or when they took paracetamol with flucloxacillin. Symptoms of metabolic acidosis may include: severe breathing difficulties, including rapid deep breathing, drowsiness, feeling of nausea (nausea) and vomiting.
In patients with asthma, hypersensitivity to salicylates (e.g. acetylsalicylic acid) may occur with paracetamol.
Available study results indicate that paracetamol administration may be a risk factor for the development of asthma and allergic diseases in children.
Paracetamol Hasco Forte and other medicines
You should tell your doctor or pharmacist about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to take.
The medicine should not be taken with centrally acting analgesics or with alcohol, as it enhances their effect. In the case of concurrent use: barbiturates, antiepileptic drugs (including glutethimide, phenobarbital, phenytoin, carbamazepine), rifampicin, the harmful effect of paracetamol on the liver is increased. Paracetamol increases the toxicity of chloramphenicol.
Prolonged use of paracetamol in high doses enhances the effect of oral anticoagulant medicines from the coumarin group.
Concomitant use of paracetamol with non-steroidal anti-inflammatory drugs (NSAIDs) increases the risk of kidney function disorders. Paracetamol used concomitantly with MAO inhibitors may cause a state of excitement and high temperature.
The absorption of paracetamol is accelerated by drugs that accelerate gastric emptying (e.g. metoclopramide, domperidone) and delayed by drugs that delay gastric emptying (e.g. cholestyramine).
Using paracetamol in combination with zidovudine may cause neutropenia. Salicylamide prolongs the elimination time of paracetamol.
Using paracetamol may be the cause of false results in some laboratory tests (e.g. blood glucose measurement).
You should inform your doctor or pharmacist if the patient is taking flucloxacillin (an antibiotic) due to the serious risk of blood and body fluid disorders (called metabolic acidosis), which must be urgently treated (see section 2).
Paracetamol Hasco Forte with alcohol
While using Paracetamol Hasco Forte, you should not drink alcohol, as it may cause liver damage.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
Like other medicines, this medicine should be used during pregnancy and breastfeeding only if necessary.
Driving and using machines
The medicine has no effect on the ability to drive vehicles and operate machines.
Paracetamol Hasco Forte contains 3.7 g of sucrose in 5 ml of suspension
This should be taken into account in patients with diabetes. If the patient has previously been diagnosed with intolerance to some sugars, they should consult a doctor before taking the medicine.
Paracetamol Hasco Forte contains sodium metabisulfite (E 223)
Sodium metabisulfite may rarely cause severe hypersensitivity reactions and bronchospasm.
Paracetamol Hasco Forte contains 7.059 mg of sodium benzoate (E 211) in 5 ml of suspension
Sodium benzoate may increase the risk of jaundice (yellowing of the skin and whites of the eyes) in newborns (up to 4 weeks of age).
Paracetamol Hasco Forte contains less than 1 mmol (23 mg) of sodium per 5 ml of suspension, which means the medicine is considered "sodium-free".
Paracetamol Hasco Forte contains d-limonene
Paracetamol Hasco Forte contains 11.579 mg of propylene glycol (E 1520) in 5 ml of suspension
Before administering the medicinal product to a child under 4 weeks of age, you should consult a doctor or pharmacist, especially if the child is taking other medicinal products containing propylene glycol or alcohol.
3. How to use Paracetamol Hasco Forte
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor or pharmacist. If you are unsure, you should consult a doctor or pharmacist.
The medicine is for oral use.
Before use, shake the bottle vigorously to obtain a uniform suspension.
Paracetamol Hasco Forte suspension contains twice the concentration of paracetamol as Paracetamol Hasco suspension.
Recommended dose:
Unless otherwise directed by a doctor, the average single dose of paracetamol is 10 mg to 15 mg per kilogram of body weight.
If necessary, the dose can be repeated, but not more often than every 4-6 hours, up to 4 times a day, i.e. a maximum of 60 mg/kilogram of body weight/day.
The following table shows an example dosage schedule for the medicine:
| Age (child's body weight) | Recommended single dose | Maximum daily dose |
| from 0 to 3 months of age (up to 4 kg) | 1.25 ml (60 mg) | 5 ml (240 mg) |
| from 4 to 8 months of age (up to 7 kg) | 2 ml (96 mg) | 8 ml (384 mg) |
| from 9 to 11 months of age (up to 8 kg) | 2.5 ml (120 mg) | 10 ml (480 mg) |
| from 1 to 2 years of age (up to 10.5 kg) | 3.25 ml (156 mg) | 13 ml (624 mg) |
| from 2 to 3 years of age (up to 13 kg) | 4 ml (192 mg) | 16 ml (768 mg) |
| from 4 to 5 years of age (up to 18.5 kg) | 6 ml (288 mg) | 24 ml (1152 mg) |
| from 6 to 8 years of age (up to 24 kg) | 7.5 ml (360 mg) | 30 ml (1440 mg) |
| from 9 to 10 years of age (up to 32 kg) | 10 ml (480 mg) | 40 ml (1920 mg) |
| from 11 to 12 years of age (up to 45.6 kg) | 14.25 ml (684 mg) | 57 ml (2736 mg) |
Without consulting a doctor, do not use for more than 3 days. Use in children under 2 years of age should be prescribed by a doctor. An oral syringe is attached to the packaging.
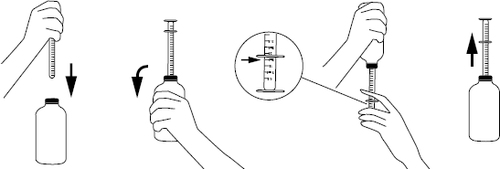
Instructions for dosing with an oral syringe:
- after unscrewing the cap, put the syringe on the plug located in the neck of the bottle,
- to fill the syringe, shake the bottle vigorously, turn it upside down, and then carefully move the syringe plunger down, drawing in the suspension in the desired amount indicated on the scale,
- turn the bottle back to its original position and carefully remove the syringe from the plug,
- place the tip of the syringe in the child's mouth and, slowly pressing the plunger, carefully empty the contents of the syringe,
- after use, close the bottle and wash and dry the syringe.
There are no special instructions for use with food.
If you feel that the effect of Paracetamol Hasco Forte is too strong or too weak, you should consult a doctor .
Using a higher dose of Paracetamol Hasco Forte than recommended
In case of taking a higher dose of the medicine than recommended, you should immediately consult a doctor or pharmacist, even if the patient feels well.
Severe poisoning may occur in children after taking 200 mg of paracetamol/kg of body weight.
Accidental or intentional overdose of paracetamol may cause symptoms such as: nausea, vomiting, excessive sweating, drowsiness, and general weakness within a few or several hours. These symptoms may resolve the next day, despite the slow development of severe liver damage, manifested by a feeling of fullness in the upper abdomen, nausea, and jaundice.
Treatment of paracetamol poisoning must be carried out in a hospital, under intensive medical care. If it has been less than an hour since taking paracetamol, you should induce vomiting and administer activated charcoal.
Missing a dose of Paracetamol Hasco Forte
Paracetamol Hasco Forte is used as needed, when symptoms occur. It should be taken according to the recommendations given in section 3. However, if a doctor recommends regular use of the medicine, you should not take a double dose to make up for a missed dose.
If you have any further doubts about using this medicine, you should consult a doctor or pharmacist .
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Rarely(in 1 to 10 patients out of 10,000):
allergic skin reactions: hives, rash, skin inflammation.
Very rarely(in less than 1 patient out of 10,000):
decreased platelet count (thrombocytopenia)
decreased white blood cell count (leukopenia, agranulocytosis)
nausea, vomiting, diarrhea
liver function disorders.
Frequency not known(cannot be estimated from the available data):
a serious disease that can cause acidification of the blood (so-called metabolic acidosis), in patients with severe disease taking paracetamol (see section 2).
Long-term use of the medicine or overdose may cause liver and kidney damage, as well as methemoglobinemia with symptoms of cyanosis (gray-blue skin discoloration).
There have been reports of very rare cases of severe skin reactions (acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, toxic epidermal necrolysis).
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181 C, 02-222 Warsaw,
phone: 22 49 21 301, fax: 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Paracetamol Hasco Forte
Store in a temperature below 25°C. The product should not be stored in an inverted position.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Shelf life after first opening: 12 months.
Medicines should not be disposed of via wastewater or household waste containers. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Paracetamol Hasco Forte contains
- The active substance of the medicine is paracetamol. 5 ml of oral suspension contains 240 mg of paracetamol.
- The other ingredients (excipients) are: sucrose, xanthan gum (E 415), citric acid monohydrate (E 330), sodium benzoate (E 211), sodium metabisulfite (E 223), natural strawberry flavor AR 2143 (contains natural flavoring substances, including d-limonene and propylene glycol), purified water.
What Paracetamol Hasco Forte looks like and what the packaging contains
A milky to light yellow suspension with a strawberry smell. The packaging contains 150 ml, 100 ml, or 85 ml of oral suspension.
A bottle made of orange glass type III with an HDPE cap, with a guarantee ring and a connector made of LDPE for an oral syringe, and an oral syringe made of LDPE/PS with a capacity of 5 ml, with a scale every 0.25 ml, in a cardboard box.
Marketing authorization holder and manufacturer
"PRZEDSIĘBIORSTWO PRODUKCJI FARMACEUTYCZNEJ HASCO-LEK" S.A.
51-131 Wrocław, ul. Żmigrodzka 242 E
Medicine information
phone: 22 742 00 22
e-mail: [email protected]
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterPrzedsiębiorstwo Produkcji Farmaceutycznej HASCO-LEK S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Paracetamol Hasco ForteDosage form: Tablets, 500 mgActive substance: paracetamolManufacturer: Farmaceutyczna Spółdzielnia Pracy "Galena"Prescription not requiredDosage form: Tablets, 300 mgActive substance: paracetamolManufacturer: Farmaceutyczna Spółdzielnia Pracy "Galena"Prescription not requiredDosage form: Tablets, 325 mgActive substance: paracetamolManufacturer: US Pharmacia Sp. z o.o.Prescription not required
Alternatives to Paracetamol Hasco Forte in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Paracetamol Hasco Forte in Spain
Alternative to Paracetamol Hasco Forte in Ukraine
Online doctors for Paracetamol Hasco Forte
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Paracetamol Hasco Forte – subject to medical assessment and local rules.















