
Pangrol 10 000

Ask a doctor about a prescription for Pangrol 10 000

How to use Pangrol 10 000
Package Leaflet: Information for the User
Pangrol 10,000,10,000 Ph.Eur. lipase, capsules
(Pancreatinum)
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
This medicine should always be taken exactly as described in the patient leaflet or as directed by your doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, consult your pharmacist.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 7-14 days there is no improvement or you feel worse, contact your doctor.
Table of Contents of the Leaflet:
- 1. What is Pangrol 10,000 and what is it used for
- 2. Important information before taking Pangrol 10,000
- 3. How to take Pangrol 10,000
- 4. Possible side effects
- 5. How to store Pangrol 10,000
- 6. Contents of the pack and other information
1. What is Pangrol 10,000 and what is it used for
What is Pangrol 10,000?
Pangrol 10,000 contains digestive enzymes (enzymes) of porcine origin (powdered pancreas, also known as pancreatin).
What is Pangrol 10,000 used for?
Pangrol 10,000 is used as a supplement to pancreatic enzymes in people with digestive disorders resulting from a lack or insufficient secretion and/or action of pancreatic enzymes in the duodenum. Such disorders may occur, for example, in the following circumstances:
- chronic pancreatitis, regardless of the cause (alcoholic, traumatic, autoimmune, drug-induced, tropical calcification, idiopathic);
- specific pancreatic dysfunction (cystic fibrosis);
- constriction of the pancreatic duct caused by, for example, a tumor or gallstones;
- liver and biliary disorders;
- previous pancreatic surgery or pancreatectomy and duodenectomy;
- accelerated intestinal passage after gastric or intestinal surgery, due to stress or inflammatory bowel disease;
- consumption of difficult-to-digest plant foods, fats, or foods to which the digestive system is not accustomed, leading to impaired absorption of nutrients and digestive disorders with accompanying loss of appetite, bloating, nausea, and diarrhea (malabsorption);
- intestinal disease;
- inflammatory bowel disease (especially Crohn's disease);
- diabetes;
- acquired immune deficiency syndrome (AIDS);
- Shwachman syndrome;
- Sjögren's syndrome.
The medicine can be used in adults (including the elderly) and children. If after 7-14 days there is no improvement or you feel worse, contact your doctor.
2. Important information before taking Pangrol 10,000
When not to take Pangrol 10,000
- if you are allergic to pancreatin, pork meat, or any of the other ingredients of this medicine (listed in section 6).
- in patients with acute pancreatitis and acute exacerbations of chronic pancreatitis, in the acute phase of the disease.
However, occasional administration of the medicine is appropriate in the phase of subsiding disease, during the expansion of the oral diet (light diet), if symptoms of persistent pancreatic dysfunction occur.
Warnings and precautions
Before taking Pangrol 10,000, discuss it with your doctor or pharmacist: in case of symptoms indicating intestinal obstruction (e.g., abdominal pain, lack of bowel movements, nausea, vomiting). Intestinal obstruction is a common complication in patients with cystic fibrosis. As a precaution, any unusual symptoms from the abdomen or stomach and intestines, or changes in existing symptoms, should be examined by a doctor to rule out intestinal damage. This applies especially to patients taking more than 10,000 Ph.Eur. lipase per kilogram of body weight per day. This medicine contains active enzymes that, if released in the mouth, for example, by chewing, can cause damage (ulcers) to the oral mucosa. Therefore, caution should be exercised, and the medicine should only be taken whole.
- j. Ph. Eur. lipase per kilogram of body weight per day.
Pangrol 10,000 and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take. Taking ready-made medicines containing pancreatic enzymes may reduce the absorption of folic acid, which means that additional folic acid may be necessary. Taking Pangrol 10,000 at the same time may weaken the effect of oral anti-diabetic medicines: acarbose and miglitol.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before taking this medicine. There is no experience with the use of the medicinal product Pangrol 10,000 in pregnant women. Only incomplete data from animal studies on the effect on pregnancy, fetal development, childbirth, and postnatal development are available. The potential risk to humans is not known. If you are pregnant or breastfeeding, you should not take Pangrol 10,000 unless your doctor considers it absolutely necessary.
Driving and using machines
Pangrol 10,000 has no influence or negligible influence on the ability to drive and use machines.
3. How to take Pangrol 10,000
This medicine should always be taken exactly as described in the leaflet or as directed by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist.
Dosage
The recommended dose is 2-4 capsules of Pangrol 10,000 per meal (corresponding to 20,000-40,000 Ph.Eur. lipase). The dosage depends on the severity of the digestive disorders. It may be necessary to take higher doses. Increasing the dosage requires prior consultation with a doctor, and its purpose should be to achieve improvement in relation to symptoms (e.g., fatty stools, stomach pain). Do not exceed the daily dose of 15,000-20,000 lipase units per kilogram of body weight. In cystic fibrosis, Pangrol 10,000 can be used in adults and children with a body weight of at least 10 kg. Unless the doctor recommends a different dosage, patients with cystic fibrosis with a body weight of at least 10 kg should take 1-2 capsules of Pangrol 10,000 per meal (equivalent to 10,000-20,000 Ph.Eur. lipase per meal). The doctor may decide to increase the dose depending on body weight and symptom severity. Do not exceed the maximum dose of 15,000-20,000 lipase units per kilogram of body weight per day.
Children and adolescents
The dosage in children is determined by the doctor.
Method of administration
Pangrol 10,000 capsules should be swallowed whole during a meal, washed down with a large amount of water. Patients who do not want to swallow the capsule whole can open it over a suitable container (e.g., a glass) and swallow the contents whole with a small amount of liquid.
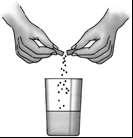
Be careful not to chew the tablets, as this may reduce the effectiveness of the medicine, and the enzymes released in the mouth may cause damage to the oral mucosa. Drink a large amount of fluid (water or juice).
Duration of treatment
The duration of treatment with Pangrol 10,000 is not limited. The length of treatment depends on the course of the disease and is determined by the doctor. If you do not feel better or feel worse, contact your doctor. If you feel that the effect of Pangrol 10,000 is too strong or too weak, consult your doctor or pharmacist.
Taking a higher dose of Pangrol 10,000 than recommended
In case of taking a higher dose of the medicine than recommended, drink a large amount of water and consult your doctor. Taking very high doses of pancreatin, especially in patients with cystic fibrosis, may lead to an increased level of uric acid in the blood (hyperuricemia) and urine (hyperuricosuria).
Missing a dose of Pangrol 10,000
Do not take a double dose to make up for a missed dose. Take the next dose as directed.
Stopping treatment with Pangrol 10,000
In case of premature termination or interruption of treatment with Pangrol 10,000, you should expect the symptoms to return. Contact your doctor. If you have any further doubts about the use of this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Pangrol 10,000 can cause side effects, although not everybody gets them.
Important side effects and symptoms to watch for
If you experience any of the following symptoms, stop taking Pangrol 10,000 and consult your doctor immediately. The doctor will decide on further action.
Very rare side effects (may affect up to 1 in 10,000 people)
Diarrhea, discomfort in the abdominal cavity, abdominal pain, nausea, vomiting. Allergic reactions, such as: skin rash, hives, sneezing, tearing, shortness of breath due to bronchospasm, shortness of breath. Allergic reactions from the gastrointestinal tract. In patients with cystic fibrosis, after taking high doses of pancreatin, narrowing of the lower parts of the intestine (in the ileocecal and ascending colon) has been reported. This can lead to intestinal obstruction (see section 2 "Warnings and precautions").
Frequency not known (cannot be estimated from the available data)
In patients with cystic fibrosis, mainly those taking high doses of pancreatin, there may be an increased excretion of uric acid in the urine. Therefore, the uric acid concentration in the urine of these patients should be monitored to prevent the formation of uric acid stones.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Post-Marketing Surveillance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw. Phone: (22) 49-21-301. Fax: (22) 49-21-309. Website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Pangrol 10,000
Keep out of the sight and reach of children. Do not use this medicine after the expiry date stated on the carton and blister or bottle after the "EXP" symbol. The expiry date refers to the last day of the month. The container should be kept tightly closed.
Storage conditions
Store in a temperature below 25°C. After opening the bottle, the medicine retains its potency for 6 months. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Pangrol 10,000 contains
The active substance is powdered porcine pancreas. One capsule contains enteric-coated mini-tablets containing powdered porcine pancreas with an activity of: Lipase 10,000 Ph.Eur. Amylase 9,000 Ph.Eur. Protease 500 Ph.Eur.
Excipients
mini-tablets:
Croscarmellose sodium
Microcrystalline cellulose
Hydrogenated castor oil
Silica, colloidal anhydrous
Magnesium stearate
30% aqueous dispersion of ethyl acrylate and methyl methacrylate copolymer (1:1), type C
Triethyl citrate
Simethicone emulsion 30%
Talc
capsule:
Gelatin
Iron oxide red (E 172)
Quinoline yellow (E 104)
Iron oxide yellow (E 172)
Indigo carmine (E 132)
Titanium dioxide (E 171)
What Pangrol 10,000 looks like and contents of the pack
Pangrol 10,000 is a hard gelatin capsule. The upper part of the capsule is light green, and the lower part is light brown. The capsules are filled with enteric-coated mini-tablets. The pack contains: a polypropylene bottle with a polyethylene cap and a desiccant, containing 20 or 50 capsules. Blister packs of aluminum (OPA/Al/PVC)/aluminum in a cardboard box. The boxes contain 20 or 50 capsules. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
BERLIN-CHEMIE AG
Glienicker Weg 125
12489 Berlin, Germany
For more detailed information, please contact the representative of the marketing authorization holder:
Berlin-Chemie/Menarini Polska Sp. z o.o.
Tel: (022) 566 21 00
Fax: (022) 566 21 01
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterBerlin-Chemie AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Pangrol 10 000Dosage form: Capsules, 10,000 Ph.Eur.U. of lipolytic activityActive substance: multienzymes (lipase, protease etc.)Prescription not requiredDosage form: Capsules, 25,000 Ph. Eur. lipase unitsActive substance: multienzymes (lipase, protease etc.)Manufacturer: Abbott Laboratories GmbH, Werk NeustadtPrescription requiredDosage form: Capsules, 35000 Ph.Eur.U. of lipolytic activityActive substance: multienzymes (lipase, protease etc.)Prescription required
Alternatives to Pangrol 10 000 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Pangrol 10 000 in Ukraina
Alternative to Pangrol 10 000 in Hiszpania
Online doctors for Pangrol 10 000
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pangrol 10 000 – subject to medical assessment and local rules.














