
Oxinador
Ask a doctor about a prescription for Oxinador

How to use Oxinador
Leaflet accompanying the packaging: patient information
Oxynador, 10 mg + 5 mg, prolonged-release tablets
Oxynador, 20 mg + 10 mg, prolonged-release tablets
Oxynador, 40 mg + 20 mg, prolonged-release tablets
Oxycodone hydrochloride + naloxone hydrochloride
You should carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Oxynador and what is it used for
- 2. Important information before taking Oxynador
- 3. How to take Oxynador
- 4. Possible side effects
- 5. How to store Oxynador
- 6. Package contents and other information
1. What is Oxynador and what is it used for
Oxynador is a prolonged-release tablet, which means that the active substances are released from the tablet over a longer period. Their effect lasts for 12 hours. These tablets can only be used in adults.
Pain relief
Oxynador is indicated for the treatment of severe pain that can only be adequately controlled with opioid analgesics.
How the tablets work to relieve pain
Oxynador contains oxycodone hydrochloride and naloxone hydrochloride as active substances. Oxycodone hydrochloride is responsible for the analgesic effect of Oxynador and is a strong opioid analgesic. The second active substance of Oxynador, naloxone hydrochloride, is intended to alleviate some of the side effects that occur during treatment with opioid analgesics.
2. Important information before taking Oxynador
When not to take Oxynador
- if the patient is allergic to oxycodone hydrochloride, naloxone hydrochloride, or any of the other ingredients of this medicine (listed in section 6),
- if the patient has breathing problems, resulting in insufficient oxygen supply to the blood and removal of carbon dioxide produced in the body (respiratory depression),
- if the patient has severe lung disease associated with airway narrowing (chronic obstructive pulmonary disease, COPD),
- if the patient has been diagnosed with a condition called cor pulmonale. In this case, the right side of the heart is enlarged due to increased pressure in the blood vessels in the lungs (e.g., as a result of COPD - see above),
- if the patient has severe asthma,
- if the patient has paralytic ileus (a type of intestinal obstruction) not caused by opioids,
- if the patient has moderate to severe liver failure.
Warnings and precautions
Before starting treatment with Oxynador, you should consult a doctor or pharmacist:
- in the case of elderly and frail patients,
- if the patient has paralytic ileus (a type of intestinal obstruction) caused by opioids,
- if the patient has kidney failure,
- if the patient has mild liver failure,
- if the patient has severe lung failure (i.e., reduced vital capacity),
- if the patient experiences frequent pauses in breathing during sleep at night (sleep apnea), which can cause excessive daytime sleepiness
- if the patient suffers from a condition characterized by frequent breathing pauses at night, which can cause sleepiness during the day (sleep apnea);
- if the patient has edema (a disorder of thyroid function characterized by dryness, decreased temperature, and swelling of the skin ("moon face"), including the face and limbs),
- if the thyroid gland does not produce enough hormones (underactive thyroid or hypothyroidism),
- if the adrenal glands do not produce enough hormones (adrenal insufficiency or Addison's disease),
- if the patient has a mental disorder with accompanying (partial) loss of touch with reality (psychosis) caused by alcohol or other substance intoxication (substances that induce psychosis),
- if the patient has symptoms related to gallstones or other bile duct disorders (bile duct diseases, gallbladder, etc.),
- if the patient has been diagnosed with benign prostatic hyperplasia (enlarged prostate),
- if the patient has a history of alcoholism or delirium tremens,
- if the patient has pancreatitis,
- if the patient has low blood pressure (hypotension),
- if the patient has high blood pressure (hypertension),
- if the patient has previously diagnosed cardiovascular disease,
- if the patient has a head injury (due to the risk of increased intracranial pressure),
- if the patient has epilepsy or a predisposition to seizures,
- if the patient is taking MAO inhibitors (used to treat depression or Parkinson's disease) or if the patient has taken them within the last two weeks, e.g., drugs containing tranylcypromine, phenelzine, isocarboxazid, moclobemide, and linezolid;
- if the patient feels drowsy or falls asleep unexpectedly;
Breathing difficulties during sleep
Oxynador may cause breathing difficulties related to sleep, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, waking up due to shortness of breath, difficulty falling asleep, or excessive daytime sleepiness. If the patient or another person observes such symptoms, they should contact a doctor. The doctor may recommend reducing the dose of the medicine.
You should consult a doctor if the above information applied to the patient at any point in the past. You should also consult a doctor if any of the above disorders develop during treatment with these tablets. The most serious consequence of opioid overdose is respiratory depression (slow and shallow breathing). This can lead to a decrease in oxygen levels in the blood, resulting in fainting.
The prolonged-release tablet should be swallowed whole, so as not to affect the slow release of oxycodone hydrochloride from the prolonged-release tablet. The tablets should not be broken, chewed, or crushed. Taking a broken, chewed, or crushed tablet can lead to the absorption of a potentially fatal dose of oxycodone hydrochloride (see "Taking a higher dose of Oxynador than recommended" in section 3).
If severe diarrhea occurs at the beginning of treatment, it may be caused by the effect of naloxone. This may indicate that bowel function is returning to normal. Such diarrhea may occur within the first 3-5 days of treatment. If diarrhea persists after 3-5 days or bothers the patient, they should contact a doctor.
In the case of switching to Oxynador after previously taking other opioid analgesics, initial withdrawal symptoms may occur, such as anxiety, sweating, and muscle pain. If the patient experiences such symptoms, special medical supervision may be necessary.
Tolerance, dependence, and addiction
This medicine contains oxycodone, which is an opioid. Repeated use of opioid medicines can lead to decreased effectiveness of the medicine (the patient becomes accustomed to it, which is called tolerance). Repeated use of Oxynador can lead to dependence and abuse, which can lead to life-threatening overdose. The risk of these side effects may increase with increasing dose and longer treatment duration.
Dependence or abuse can cause the patient to feel that they have lost control over the dose of the medicine they take or how often they take it. The patient may feel that they need to continue taking the medicine, even if it no longer helps to relieve pain.
The risk of dependence on the medicine or addiction varies from person to person. The risk of addiction to Oxynador may be higher if:
- the patient or someone in their family has previously abused or been dependent on alcohol,
- prescription drugs or narcotics;
- the patient smokes;
- the patient has had mood problems (depression, anxiety, or personality disorders) or has been treated
- by a psychiatrist for other mental illnesses.
If the patient notices any of the following symptoms while taking Oxynador, it may indicate that they are developing tolerance to the medicine or are becoming dependent:
- The patient needs to take the medicine for a longer period than the doctor recommended.
- The patient must take a higher dose than recommended.
- The patient uses the medicine for reasons other than those recommended by the doctor, such as "to feel calm" or "to help fall asleep".
- The patient has repeatedly tried to stop or control the use of the medicine but has failed.
- The patient feels unwell after stopping the medicine and feels better after taking it again ("withdrawal symptoms").
If any of these symptoms are observed, the patient should contact a doctor to discuss the best treatment path, including when to stop taking the medicine and how to safely stop treatment (see section 3 "Stopping Oxynador treatment").
The patient should contact a doctor if they experience severe abdominal pain that may radiate to the back, nausea, vomiting, or fever, as these may be symptoms related to pancreatitis and bile duct disorders.
In the case of patients with cancer-related metastases to the peritoneum or intestinal obstruction in advanced stages of gastrointestinal cancer, the doctor should be informed of this fact.
If surgery is necessary, the doctors should be informed about the use of Oxynador.
Like other opioids, oxycodone can affect the normal production of hormones in the body, such as cortisol or sex hormones, especially if the patient has taken high doses of the medicine for a long time. If the patient experiences symptoms that persist, such as nausea (including vomiting), loss of appetite, fatigue, weakness, dizziness, changes in menstrual cycle, impotence, infertility, or decreased sex drive, they should discuss this with their doctor, as hormone level monitoring may be necessary.
This medicine may increase sensitivity to pain, especially at high doses. The patient should inform their doctor if this happens. It may be necessary to reduce the dose or change the medicine.
A residue of the prolonged-release tablet may be visible in the stool. The patient should not be concerned, as the active substances (oxycodone hydrochloride and naloxone hydrochloride) have already been released and absorbed during the passage of the tablet through the digestive system.
Incorrect use of Oxynador tablets
The tablets are not suitable for treating withdrawal symptoms.
Never misuse Oxynador, especially if you are dependent on drugs. In people addicted to substances such as heroin, morphine, or methadone, misusing these tablets can cause severe withdrawal symptoms, as they contain naloxone. Pre-existing withdrawal symptoms may worsen.
The tablets should not be misused by dissolving and injecting the contents of the tablets (e.g., into blood vessels). The tablets contain talc, which can cause local tissue breakdown (necrosis) and changes in lung tissue (pulmonary granulomas). Such misuse can also lead to other serious consequences and even death.
The use of these tablets may result in a positive test for stimulants (doping).
Using Oxynador as a stimulant can be life-threatening.
Oxynador and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
The risk of side effects increases if the patient is taking antidepressants (such as citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, venlafaxine). These medicines can interact with oxycodone, which may cause the patient to experience symptoms such as involuntary, rhythmic muscle contractions, including muscles that control eye movements, agitation, excessive sweating, tremors, increased reflexes, increased muscle tone, and elevated body temperature above 38°C.
If these symptoms occur, the patient should contact their doctor.
Concomitant use of opioids, including oxycodone hydrochloride, and sedatives, such as benzodiazepines or similar medicines, increases the risk of drowsiness, breathing difficulties (respiratory failure), coma, and can be life-threatening. Therefore, concomitant use of these medicines should only be considered when other treatment options are not possible.
If the doctor prescribes Oxynador together with sedatives, they should limit the dose and duration of concomitant use.
The patient should inform their doctor about all sedatives they are taking and strictly follow the doctor's dosage instructions. It may be helpful to inform friends or relatives to be aware of the above symptoms. If such symptoms occur, the patient should contact their doctor.
Examples of sedatives or medicines with a similar effect are:
- other strong painkillers (opioids);
- medicines used to treat epilepsy, pain, and anxiety, such as gabapentin and pregabalin;
- sleeping pills and sedatives (including benzodiazepines and anxiolytics)
- medicines used to treat depression;
- medicines used to treat allergies, motion sickness, or nausea (antihistamines or antiemetics);
- medicines used to treat mental or psychiatric disorders (antipsychotics containing phenothiazines, neuroleptics);
- muscle relaxants;
- medicines used to treat Parkinson's disease.
In the case of concomitant use of these tablets and other medicines, the effect of both the tablets and the medicines listed below may change.
The patient should inform their doctor about taking medicines such as:
- medicines that reduce blood clotting (coumarin derivatives), as the blood clotting time may be prolonged or shortened;
- macrolide antibiotics (e.g., clarithromycin, erythromycin, or telithromycin);
- azole antifungal medicines (such as ketoconazole, voriconazole, itraconazole, or posaconazole);
- protease inhibitors used to treat HIV (e.g., ritonavir, indinavir, nelfinavir, and saquinavir);
- cimetidine (a medicine for stomach ulcers, indigestion, or heartburn);
- rifampicin (used to treat tuberculosis);
- carbamazepine (used to treat seizures, strokes, or certain types of pain);
- phenytoin (used to treat seizures, strokes, or certain types of pain);
- St. John's Wort (also known as Hypericum perforatum);
- quinidine (a medicine for heart rhythm disorders).
No interaction is expected between Oxynador and paracetamol, acetylsalicylic acid, or naltrexone.
Oxynador with food, drink, and alcohol
Drinking alcohol while taking Oxynador may cause drowsiness or increase the risk of serious side effects, such as shallow breathing with a risk of apnea and loss of consciousness. It is not recommended to drink alcohol while taking Oxynador. The patient should avoid drinking grapefruit juice while taking these tablets.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before taking this medicine.
Pregnancy
The patient should avoid taking these tablets during pregnancy if possible. Long-term use of prolonged-release tablets during pregnancy may cause withdrawal symptoms in the newborn. If oxycodone hydrochloride is used during delivery, the newborn may experience respiratory depression (slow and shallow breathing).
Breastfeeding
The patient should stop breastfeeding while taking these tablets. Oxycodone hydrochloride passes into breast milk. It is not known whether naloxone hydrochloride also passes into breast milk. Therefore, the risk to the breastfed infant cannot be excluded if the mother takes Oxynador long-term.
Driving and using machines
Oxynador may impair the ability to drive and use machines. Such effects can be expected especially at the beginning of treatment with Oxynador, after each dose increase, or when switching to another medicine. However, when the patient has been taking a fixed dose of Oxynador for a long time, these disturbances disappear.
Oxynador has been associated with drowsiness and sudden sleep episodes. If the patient experiences this side effect, they should not drive or operate machinery. The patient should discuss this with their doctor if they experience such side effects.
The patient should consult their doctor about the possibility of driving or operating machinery.
Oxynador contains lactose
The medicine contains lactose (milk sugar). If the patient has previously been diagnosed with intolerance to some sugars, they should contact their doctor before taking these tablets.
3. How to take Oxynador
This medicine should always be taken exactly as the doctor has instructed. In case of doubts, the patient should consult a doctor or pharmacist.
Before starting treatment and regularly during treatment, the doctor will discuss with the patient the expected symptoms of taking Oxynador, when and for how long the patient should take it, when the patient should contact the doctor, and when the patient should stop taking Oxynador (see "Stopping Oxynador treatment").
Oxynador is available in the form of prolonged-release tablets, which means that the active substances are released over a longer period. Their effect lasts for 12 hours.
The prolonged-release tablets should be swallowed whole, without chewing, with a sufficient amount of liquid (½ glass of water). The tablets can be taken with or without food. The tablets should be taken every 12 hours, according to the established treatment plan (e.g., in the morning at 8:00, in the evening at 20:00). The prolonged-release tablets should not be broken, chewed, or crushed (see section 2 "Warnings and precautions").
This only applies to single-dose blisters with a child-resistant closure:
Oxynador is available in single-dose blisters with a child-resistant closure. The prolonged-release tablet should be removed from the blister as follows:
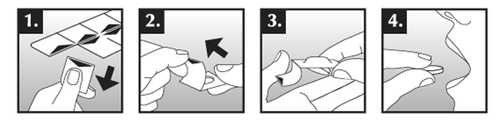
- 1. Hold the edge of the blister and separate one unit from the rest of the blister by gently tearing it along the perforation.
- 2. Pull the edge of the foil and completely remove the foil.
- 3. Push the prolonged-release tablet out onto your hand.
- 4. Swallow the prolonged-release tablet whole, with a sufficient amount of liquid, with or without food.
Duration of treatment
As a rule, the patient should not take these tablets for longer than necessary. If the patient is undergoing long-term treatment with these tablets, the treating doctor should regularly check if the patient still needs them.
Taking a higher dose of Oxynador than recommended
If the patient has taken a higher dose of the tablets than recommended, they should immediately contact a doctor.
Overdose of the medicine may be characterized by:
- constricted pupils,
- slow and shallow breathing (respiratory depression),
- a state similar to narcotic intoxication (drowsiness leading to loss of consciousness),
- decreased muscle tone (hypotonia),
- slow heart rate,
- decreased blood pressure,
- brain disorders (toxic leukoencephalopathy).
In severe cases, loss of consciousness (coma), water retention in the lungs, and circulatory collapse may occur, which can lead to death in some cases.
The patient should avoid activities that require increased attention, such as driving vehicles.
Missing a dose of Oxynador
or taking a lower dose than prescribed may result in inadequate pain relief.
If the patient has forgotten to take a dose, they should follow the instructions:
- If there are 8 hours or more until the next dose: the patient should take the missed dose immediately and continue taking the medicine according to the established schedule.
- If there are less than 8 hours until the next dose: the patient should take the missed dose. Then, they should wait 8 hours before taking the next dose. The patient should try to return to the original dosing schedule (e.g., in the morning at 8:00, in the evening at 20:00). The patient should not take more than one dose in 8 hours.
The patient should not take a double dose to make up for a missed dose.
Stopping Oxynador treatment
The patient should not stop taking Oxynador without consulting a doctor.
If treatment is no longer necessary, the patient should gradually reduce the daily dose after consulting a doctor. This can help avoid withdrawal symptoms, such as anxiety, restlessness, sweating, and muscle pain.
If the patient has any further doubts about taking these tablets, they should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Oxynador can cause side effects, although not everybody gets them.
Important information about side effects and what to do if they occur:
If any of the following side effects occur, the patient should immediately contact their doctor.
Slow and shallow breathing (respiratory depression) is the main risk associated with opioid overdose. It occurs mainly in elderly and frail patients. Opioids can also cause a significant drop in blood pressure in sensitive patients.
The following side effects have been observed in patients treated for pain:
- abdominal pain
- constipation
- diarrhea
- dry mouth
- indigestion
- vomiting
- nausea
- bloating (gas)
- decreased appetite up to loss of appetite
- dizziness or "spinning" sensation
- headache
- hot flashes
- fatigue or exhaustion
- feeling of unusual weakness
- itching
- skin reactions, rash
- sweating
- dizziness
- sleep disorders
- sleepiness
Uncommon(may affect up to 1 in 100 people):
- abdominal bloating
- abnormal thinking
- anxiety
- disorientation
- depression
- irritability
- chest tightness, especially if there is coronary heart disease
- decreased blood pressure
- withdrawal symptoms, e.g., agitation
- fainting
- feeling of lack of energy
- thirst
- taste disorders
- palpitations
- biliary colic
- chest pain
- general malaise
- pain
- swelling of hands, feet, and ankles
- concentration disorders
- speech disorders
- tremors
- breathing difficulties
- restlessness, especially motor
- chills
- increased liver enzyme activity
- increased blood pressure
- decreased sex drive
- common cold
- cough
- hypersensitivity/allergic reactions
- weight loss
- accidents
- increased urination
- muscle spasms
- muscle twitching
- muscle pain
- vision disorders
- seizures (especially in people with a history of seizure disorders or predisposition to seizures)
Rare(may affect up to 1 in 1,000 people):
- increased heart rate
- addiction to the medicine
- changes in teeth
- weight gain
- yawning
Unknown(frequency cannot be estimated from the available data):
- aggression
- euphoric state
- excessive sleepiness
- erectile dysfunction
- nightmares
- hallucinations
- shallow breathing
- urination disorders
- tingling sensation (numbness and tingling)
- belching
- sleep apnea (pauses in breathing during sleep).
Known side effects of the active substance oxycodone hydrochloride, if not in combination with naloxone hydrochloride:
Oxycodone may cause breathing difficulties (respiratory depression), pinpoint pupils, bronchial spasms, and smooth muscle spasms, as well as inhibition of the cough reflex.
Common(may affect up to 1 in 10 people):
- mood and personality changes (e.g., depression, feeling extremely happy)
- decreased activity
- increased activity
- urination disorders
- hiccups
Uncommon(may affect up to 1 in 100 people):
- concentration disorders
- migraines
- increased muscle tone
- involuntary muscle contractions
- abnormal bowel function (intestinal obstruction)
- dry skin
- tolerance to the medicine
- decreased sensitivity to pain and touch
- coordination disorders
- voice changes (hoarseness)
- fluid retention
- hearing disorders
- mouth ulcers
- swallowing difficulties
- toothache
- perception disorders (e.g., hallucinations, derealization)
- skin redness
- dehydration
- agitation
- decreased sex hormone levels, which can affect sperm production in men or the menstrual cycle in women
Rare(may affect up to 1 in 1,000 people):
- itchy rash (hives)
- infections, such as herpes (which can cause blisters around the mouth or genitals)
- increased appetite
- black (tarry) stools
- bleeding gums
Unknown(frequency cannot be estimated from the available data):
- acute generalized allergic reactions (anaphylactic reactions)
- increased sensitivity to pain
- amenorrhea
- neonatal withdrawal syndrome
- bile duct disorders: disorders affecting the bile duct sphincter, which can cause severe abdominal pain (bile duct sphincter disorders)
- tooth decay
Reporting side effects
If side effects occur, including any side effects not listed in the leaflet, the patient should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Oxynador
The medicine should be stored out of sight and reach of children.
The patient should not use this medicine after the expiry date stated on the packaging after the abbreviation "EXP". The expiry date refers to the last day of the specified month.
The batch number is stated on the packaging after the abbreviation "Lot".
The patient should not store the medicine at temperatures above 30°C.
The patient should store the medicine in its original packaging to protect it from moisture.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Oxynador contains
- The active substances of Oxynador are oxycodone hydrochloride and naloxone hydrochloride. 10 mg + 5 mg, prolonged-release tablets Each prolonged-release tablet contains 10 mg of oxycodone hydrochloride, equivalent to 9 mg of oxycodone, and 5 mg of naloxone hydrochloride as 5.45 mg of naloxone hydrochloride dihydrate, equivalent to 4.5 mg of naloxone. 20 mg + 10 mg, prolonged-release tablets Each prolonged-release tablet contains 20 mg of oxycodone hydrochloride, equivalent to 18 mg of oxycodone, and 10 mg of naloxone hydrochloride as 10.9 mg of naloxone hydrochloride dihydrate, equivalent to 9 mg of naloxone. 40 mg + 20 mg, prolonged-release tablets Each prolonged-release tablet contains 40 mg of oxycodone hydrochloride, equivalent to 36 mg of oxycodone, and 20 mg of naloxone hydrochloride as 21.8 mg of naloxone hydrochloride dihydrate, equivalent to 18 mg of naloxone.
- Other ingredients are hydroxypropyl cellulose, ethyl cellulose, glycerol distearate, lactose monohydrate, talc, and magnesium stearate, and in the tablet coating, polyvinyl alcohol, titanium dioxide (E 171), macrogol 3350, talc, red iron oxide (E 172) - only in 20 mg + 10 mg tablets, and yellow iron oxide (E 172) - only in 40 mg + 20 mg tablets. See section 2 "Oxynador contains lactose".
What Oxynador looks like and contents of the pack
10 mg + 5 mg, prolonged-release tablets (tablets): white, oval, slightly convex on both sides, prolonged-release tablets with "10" embossed on one side of the tablet (dimensions: 9.5 mm x 4.5 mm)
20 mg + 10 mg, prolonged-release tablets (tablets): light pink, oval, slightly convex on both sides, prolonged-release tablets with "20" embossed on one side of the tablet (dimensions: 9.5 mm x 4.5 mm)
40 mg + 20 mg, prolonged-release tablets (tablets): brown-yellow, capsule-shaped, slightly convex on both sides, prolonged-release tablets with "40" embossed on one side of the tablet (dimensions: 14.0 mm x 6.0 mm)
10 mg + 5 mg:
Packaging:10, 14, 20, 28, 30, 50, 56, 60, 90, 98, 100, or 112 prolonged-release tablets in child-resistant blisters, in a cardboard box.
20 mg + 10 mg, 40 mg + 20 mg:
Packaging:10, 20, 28, 30, 50, 56, 60, 90, 98, 100, or 112 prolonged-release tablets in child-resistant blisters, in a cardboard box.
This only applies to single-dose blisters with a child-resistant closure:
10 mg + 5 mg:
Packaging:10x1, 14x1, 20x1, 28x1, 30x1, 50x1, 56x1, 60x1, 90x1, 98x1, 100x1, or 112x1 prolonged-release tablet in single-dose child-resistant blisters, in a cardboard box.
20 mg + 10 mg, 40 mg + 20 mg:
Packaging:10x1, 20x1, 28x1, 30x1, 50x1, 56x1, 60x1, 90x1, 98x1, 100x1, or 112x1 prolonged-release tablet in single-dose child-resistant blisters, in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder
KRKA, d.d., Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Slovenia
Manufacturer
KRKA, d.d., Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Slovenia
TAD Pharma GmbH, Heinz-Lohmann-Straße 5, 27472 Cuxhaven, Germany
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
To obtain more detailed information on this medicinal product, the patient should contact the local representative of the marketing authorization holder:
KRKA-POLSKA Sp. z o.o.
ul. Równoległa 5
02-235 Warsaw
Phone: 22 57 37 500
Date of last revision of the leaflet:08.08.2024
| Belgium, Germany | Oxycodon/Naloxon Krka |
| Bulgaria | Адолакс |
| Czech Republic, Estonia | Noldoxen |
| Denmark, Finland, Sweden | Oxycodone/Naloxone Krka |
| Ireland | Nolxado |
| Croatia, Slovenia, Slovakia | Adolax |
| Hungary, Latvia | Oxynador |
| Lithuania, Romania | Dolnada |
| Portugal | Oxicodona + Naloxona TAD |
| United Kingdom (Northern Ireland) | Oxycodone hydrochloride/Naloxone hydrochloride |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterKrka, d.d., Novo mesto TAD Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OxinadorDosage form: Tablets, 5 mg + 2.5 mgActive substance: oxycodone and naloxonePrescription requiredDosage form: Tablets, 10 mg + 5 mgActive substance: oxycodone and naloxonePrescription requiredDosage form: Tablets, 20 mg + 10 mgActive substance: oxycodone and naloxonePrescription required
Alternatives to Oxinador in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oxinador in Spain
Online doctors for Oxinador
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oxinador – subject to medical assessment and local rules.














