
Oxicodon Stada
Ask a doctor about a prescription for Oxicodon Stada

How to use Oxicodon Stada
Leaflet accompanying the packaging: patient information
Oxycodon Stada, 5 mg, hard capsules
Oxycodon Stada, 10 mg, hard capsules
Oxycodon Stada, 20 mg, hard capsules
Intended for adults and adolescents over 12 years of age.
Oxycodoni hydrochloridum
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Oxycodon Stada and what is it used for
- 2. Important information before taking Oxycodon Stada
- 3. How to take Oxycodon Stada
- 4. Possible side effects
- 5. How to store Oxycodon Stada
- 6. Contents of the packaging and other information
1. What is Oxycodon Stada and what is it used for
Oxycodon Stada is a centrally acting, strong pain reliever from the opioid group.
Oxycodon Stada is used to treat severe pain that can only be properly treated with opioid pain relievers.
Intended for adults and adolescents over 12 years of age.
2. Important information before taking Oxycodon Stada
When not to take Oxycodon Stada
Warnings and precautions
Before starting treatment with Oxycodon Stada, discuss it with your doctor or pharmacist.
The patient should consult their doctor if any of these conditions apply or have applied in the past.
Tolerance, dependence, and addiction
Oxycodon Stada has a primary potential for dependence. During long-term use, tolerance to its effect may develop, and it may be necessary to take increasingly larger doses to maintain the desired pain control.
Chronic use of Oxycodon Stada may lead to physical dependence, and sudden cessation of treatment may cause withdrawal symptoms. When the patient no longer needs therapy with Oxycodon Stada, it may be advisable to gradually reduce the dose to avoid withdrawal symptoms.
This medicine contains oxycodone, which is an opioid. Repeated use of opioid pain relievers can lead to reduced efficacy (the body gets used to it, which is called tolerance). Repeated use of Oxycodon Stada may lead to dependence, abuse, and addiction, which can lead to life-threatening overdose. The risk of these side effects may be higher when using a higher dose for a longer period.
Dependence or addiction can lead to the patient being unable to control how much medicine they should take or how often they should take it. The patient may feel the need to take the medicine even if it does not relieve the pain.
The risk of developing dependence or addiction varies from person to person. The risk of addiction or dependence on Oxycodon Stada may be higher if:
- the patient or a family member has ever abused or been dependent on alcohol, prescription drugs, or street drugs ("addiction")
- the patient is a smoker
- the patient has ever had mood disorders (depression, anxiety, or personality disorder) or has been treated by a psychiatrist for other mental disorders.
If the patient notices any of the following symptoms while taking Oxycodon Stada, it may indicate that they are developing dependence or addiction:
- need to take the medicine for longer than prescribed by the doctor
- need to take a higher dose than prescribed
- taking the medicine for reasons other than prescribed, such as "to calm down" or "to help fall asleep"
- repeated, unsuccessful attempts to stop or reduce the use of the medicine
- feeling unwell after stopping the use of the medicine and improving after resuming it (withdrawal effect). If the patient has observed any of these symptoms, they should contact their doctor to discuss the best treatment plan, including the right time and safe way to stop treatment (see section 3 "Stopping Oxycodon Stada").
Rarely, increased sensitivity to pain may develop, which cannot be relieved by increasing the dose of the medicine. In such cases, the doctor may reduce the dose or use alternative opioid medication.
Oxycodon Stada should not be used before surgery or within 24 hours after surgery.
Under no circumstances should the contents of the capsules be injected, as this can lead to serious side effects that can be fatal.
During treatment with these capsules, hormonal changes may occur. The doctor may want to monitor these changes.
Consequences of abuse for doping purposes
Taking Oxycodon Stada may result in positive doping test results.
Taking Oxycodon Stada as a doping agent can pose a risk to health.
Sleep-related breathing disorders
Oxycodon Stada may cause sleep-related breathing disorders, such as sleep apnea (pauses in breathing during sleep) and hypoxemia during sleep (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to shortness of breath, difficulty staying asleep, or excessive daytime sleepiness. If the patient or another person notices such symptoms, they should contact their doctor. The doctor may consider reducing the dose.
Children
The safety and efficacy of Oxycodon Stada in children under 12 years of age have not been established. Therefore, Oxycodon Stada is not recommended for children under 12 years of age.
Oxycodon Stada with food, drink, and alcohol
Consuming alcohol while taking Oxycodon Stada may cause drowsiness or increase the risk of serious side effects, such as shallow breathing with the risk of respiratory arrest and loss of consciousness. It is not recommended to consume alcohol while taking Oxycodon Stada.
Grapefruit juice may inhibit the metabolism of oxycodone, thereby increasing its effect. Therefore, patients should avoid consuming grapefruit juice while taking Oxycodon Stada.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking this medicine.
Pregnancy
Oxycodon Stada should not be used during pregnancy unless absolutely necessary.
There are limited data on the use of oxycodone in pregnant women. Oxycodone passes through the placenta into the fetal bloodstream.
Long-term use of oxycodone during pregnancy may cause withdrawal symptoms in newborns. Use of oxycodone during delivery may cause breathing problems (respiratory depression) in the newborn.
Breastfeeding
Breastfeeding should be stopped during treatment with Oxycodon Stada. Oxycodone passes into breast milk and may affect the breastfed child, especially after repeated doses.
Driving and using machines
Oxycodone impairs alertness and reaction ability to such an extent that the ability to drive vehicles and operate machines is impaired or completely inhibited. In the case of established therapy, a complete ban on driving vehicles or operating machines may not be necessary. The attending physician must assess individual cases. The patient should discuss with their doctor whether and under what conditions they can drive a vehicle or operate machines.
Oxycodon Stada contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per capsule, which means it is considered "sodium-free".
3. How to take Oxycodon Stada
This medicine should always be taken exactly as prescribed by the doctor. In case of doubts, consult the doctor or pharmacist.
Before starting treatment and regularly during treatment, the doctor will discuss with the patient what to expect from Oxycodon Stada, when and how long to take it, when to contact the doctor, and when to stop taking it (see also "Stopping Oxycodon Stada").
Adults and adolescents over 12 years of age.
The usual initial dose is 5 mg of oxycodone hydrochloride every 6 hours. However, the doctor will prescribe the appropriate dose and determine the required frequency of administration to treat the pain.
If the patient still experiences pain while taking this medicine, they should discuss it with their doctor.
Adults with impaired renal or hepatic function
The usual initial dose is half the recommended dose for adults. The doctor will prescribe the appropriate dose based on the patient's clinical situation.
Use in children
Oxycodon Stada is not recommended for children under 12 years of age.
Method of administration
Oral administration
Oxycodon Stada should be swallowed whole, with a sufficient amount of liquid.
Oxycodon Stada can be taken with or without food.
Oxycodon Stada should not be taken with alcoholic beverages.
Instructions for using child-resistant, perforated single-dose blisters:
- 1. Do not push the capsule directly out of the blister.
- 2. Separate one cell of the blister along the perforation.
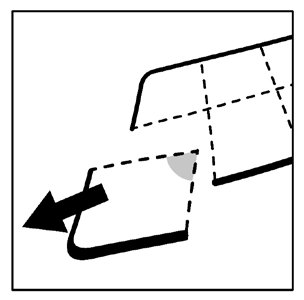
- 3. Carefully remove the protection to open the cell and remove the capsule from the cell.
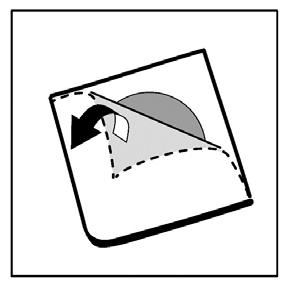
Taking a higher dose of Oxycodon Stada than prescribed
In case of taking a higher dose of Oxycodon Stada than prescribed or accidental ingestion of the capsules by someone else, the patient should immediately inform their doctor or local toxicology center.
Overdose may lead to:
constriction of the pupils
respiratory depression
muscle weakness
drowsiness and decreased blood pressure
brain disorders (toxic leukoencephalopathy).
In severe cases, it may cause circulatory collapse, lack of mental and physical activity, loss of consciousness, slowed heart rate, and fluid accumulation in the lungs.
Abuse of high doses of strong opioids, such as oxycodone, can cause death. Under no circumstances should the patient expose themselves to situations requiring increased concentration, such as driving a vehicle.
Missing a dose of Oxycodon Stada
In case of missing a dose, the patient should take the next dose as soon as possible and then take the medicine as prescribed. The patient should not take two doses within 4 hours. The patient should not take a double dose to make up for the missed dose.
Stopping Oxycodon Stada
The patient should not stop taking Oxycodon Stada without informing their doctor.
When the patient no longer needs therapy with Oxycodon Stada, it may be advisable to gradually reduce the dose to avoid withdrawal symptoms.
In case of any further doubts about the use of this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Oxycodon Stada can cause side effects, although not everybody gets them.
Important side effects or symptoms to know about and actions to take if they occur:
If any of the following side effects occur, the patient should stop taking Oxycodon Stada and contact their doctor immediately.
Respiratory depression is the most significant risk associated with opioids, and its occurrence is most likely in elderly or weakened patients.
As a result, in predisposed patients, opioids can cause significant drops in blood pressure.
In addition, oxycodone may cause constriction of the pupils, bronchospasm, and smooth muscle spasm, as well as inhibit the cough reflex.
Other possible side effects
Very common (may affect more than 1 in 10 people):
- sedation (drowsiness to sleepiness);
- dizziness;
- headache;
- constipation;
- nausea (nausea);
- vomiting;
- itching.
Common (may affect up to 1 in 10 people):
- decreased appetite, loss of appetite;
- severe mental side effects such as:
- mood changes (e.g., generalized fear, depression);
- changes in activity (mainly sedation, sometimes with accompanying fatigue, occasionally with increased nervousness and sleep disturbances);
- changes in behavior (thought process disorders, confusion);
- tremors;
- wheezing, shortness of breath, hiccups;
- dry mouth, abdominal pain, diarrhea, indigestion;
- rash, excessive sweating;
- urinary retention;
- weakness;
Uncommon (may affect up to 1 in 100 people):
- allergic reactions;
- inappropriate antidiuretic hormone secretion;
- dehydration;
- perception changes, such as depersonalization and seeing, hearing, or feeling things that do not exist (hallucinations), decreased sex drive, anxiety, extreme emotional behavior, feeling of great happiness, drug dependence (see section 2);
- increased or decreased muscle tone, coordination disorders, involuntary muscle contractions, seizures; particularly in patients with epilepsy or a history of seizures, increased tone and difficulty in stretching muscles, speech disorders, fainting, tingling or prickling (paresthesia), impaired touch sensation (hypoesthesia), migraine, taste changes, memory loss;
- lacrimation disorders, pupil constriction, vision disorders;
- abnormal hearing (hyperacusis), feeling of dizziness or spinning;
- rapid heartbeat, being aware of heartbeat;
- vasodilation;
- breathing difficulties, cough, sore throat, runny nose, voice changes;
- swallowing difficulties, oral ulcers, gingivitis, oral inflammation, bloating, belching;
- increased liver enzyme activity;
- dry skin;
- urination difficulties;
- impotence;
- low hormone levels in the blood ("hypogonadism", observed in blood tests);
- pain (e.g., chest pain), chills, excessive fluid in the tissues (edema), malaise, physical dependence including withdrawal symptoms, tolerance to the medicine requiring increased dosing to maintain the effect, craving;
- injuries caused by accidents.
Rare (may affect up to 1 in 1,000 people):
- herpes simplex (skin and mucous membrane disease);
- lymph node disease (lymphadenopathy);
- increased appetite;
- low blood pressure, dizziness when standing up from a sitting or lying position;
- gum bleeding, black stools, discoloration and tooth damage;
- pruritic rash (hives), increased sensitivity to light (photosensitivity);
- muscle spasms;
- blood in the urine (hematuria);
- changes in body weight (decrease or increase), inflammation of the subcutaneous connective tissue.
Very rare (may affect up to 1 in 10,000 people):
- exfoliative rash (exfoliative dermatitis).
Frequency not known (cannot be estimated from the available data):
- severe allergic reactions (anaphylactic reactions);
- aggressive behavior;
- increased sensitivity to pain, which cannot be relieved by increasing the dose of the medicine;
- tooth decay;
- abdominal pain on the right side, itching, and jaundice caused by gallbladder inflammation;
- absence of menstruation;
- long-term use of Oxycodon Stada during pregnancy may cause life-threatening withdrawal symptoms in the newborn. Symptoms in infants to watch out for include irritability, hyperactivity, and sleep disturbances, high-pitched crying, trembling, vomiting, diarrhea, and failure to gain weight
- sleep apnea (pauses in breathing during sleep).
Measures to take:
If the patient observes any of the side effects listed above, the doctor will take appropriate action. Constipation, as a side effect, can be prevented by a diet rich in fiber and increased fluid intake. If the patient experiences nausea or vomiting, the doctor will prescribe an appropriate medicine.
Reporting side effects
If side effects occur, including any side effects not listed in the leaflet, the patient should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products,
Jerozolimskie Avenue 181C
02-222 Warsaw
phone: +48 22 49 21 301
fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Oxycodon Stada
The medicine should be stored out of sight and reach of children. This medicine should be stored in a closed and secure place, to which other people do not have access. It can be very harmful and may cause death in a person it was not prescribed for.
Do not use this medicine after the expiry date stated on the blister/label or carton after EXP. The expiry date refers to the last day of the specified month.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Oxycodon Stada contains
- The active substance of the medicine is oxycodone hydrochloride. Oxycodon Stada, 5 mg, hard capsules: Each hard capsule contains 5 mg of oxycodone hydrochloride, equivalent to 4.5 mg of oxycodone. Oxycodon Stada, 10 mg, hard capsules: Each hard capsule contains 10 mg of oxycodone hydrochloride, equivalent to 9 mg of oxycodone. Oxycodon Stada, 20 mg, hard capsules: Each hard capsule contains 20 mg of oxycodone hydrochloride, equivalent to 18 mg of oxycodone.
- Other ingredients are: Excipients:microcrystalline cellulose, magnesium stearate. Capsule shell:gelatin, water, sodium lauryl sulfate, titanium dioxide (E 171), yellow iron oxide (E 172), red iron oxide (E 172), indigo carmine (E 132). Printing ink:shellac, black iron oxide (E 172), potassium hydroxide.
What Oxycodon Stada looks like and contents of the pack
Oxycodon Stada, 5 mg:
Hard capsules, 14.4 mm in length, with a dark pink body marked with "5" and a brown cap marked with "OXY".
Oxycodon Stada, 10 mg:
Hard capsules, 14.4 mm in length, with a white body marked with "10" and a brown cap marked with "OXY".
Oxycodon Stada, 20 mg:
Hard capsules, 14.4 mm in length, with a light pink body marked with "20" and a brown cap marked with "OXY".
Pack sizes:
10, 14, 28, 30, or 56 hard capsules
Not all pack sizes may be marketed.
Marketing authorization holder
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
Germany
Manufacturer/Importer:
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
Germany
Balkanpharma-Dupnitsa AD
3, Samokovsko Shosse Street
2600 Dupnitsa
Bulgaria
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Czech Republic:
OXYKODON STADA
Germany:
Oxycodon AL 5 mg Hartkapseln
Oxycodon AL 10 mg Hartkapseln
Oxycodon AL 20 mg Hartkapseln
Finland:
Oxycodone STADA 5 mg kapseli, kova
Oxycodone STADA 10 mg kapseli, kova
Oxycodone STADA 20 mg kapseli, kova
Poland:
Oxycodon Stada
Date of last revision of the leaflet: 03/2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBalkanpharma-Dupnitsa AD STADA Arzneimittel AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Oxicodon Stada
Alternatives to Oxicodon Stada in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oxicodon Stada in Ukraine
Alternative to Oxicodon Stada in Spain
Online doctors for Oxicodon Stada
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oxicodon Stada – subject to medical assessment and local rules.














