How to use Oxaliplatin Kabi
Package Leaflet: Information for the User
Oxaliplatin Kabi, 5 mg/ml, Concentrate for Solution for Infusion
Oxaliplatinum
Read All of This Leaflet Carefully Before You Start Using This Medicine.
Keep This Leaflet. You May Need to Read It Again.
If You Have Any Further Questions, Ask Your Doctor, Pharmacist, or Nurse.
- If You Experience Any Side Effects, Tell Your Doctor, Pharmacist, or Nurse. See Section 4.
Table of Contents
- 1. What Oxaliplatin Kabi Is and What It Is Used For
- 2. Important Information Before You Use Oxaliplatin Kabi
- 3. How to Use Oxaliplatin Kabi
- 4. Possible Side Effects
- 5. How to Store Oxaliplatin Kabi
- 6. Contents of the Pack and Other Information
1. What Oxaliplatin Kabi Is and What It Is Used For
The Active Substance of Oxaliplatin Kabi, Concentrate for Solution for Infusion, Is Oxaliplatin.
Oxaliplatin Is Used to Treat Colorectal Cancer (Stage III Colon Cancer After Complete Resection of the Primary Tumor, Metastatic Colorectal Cancer). Oxaliplatin Is Used in Combination with Other Anti-Cancer Medicines Such as 5-Fluorouracil and Folinic Acid.
Oxaliplatin Kabi Is an Anti-Cancer Medicine That Contains Platinum.
2. Important Information Before You Use Oxaliplatin Kabi
When Not to Use Oxaliplatin Kabi:
- If You Are Allergic to Oxaliplatin or Any of the Other Ingredients of This Medicine (Listed in Section 6);
- During Breast-Feeding;
- If You Have a Reduced Number of Blood Cells;
- If You Have Tingling and Numbness of the Fingers and Toes and Difficulty Performing Precise Tasks Such as Buttoning;
- If You Have Severe Kidney Impairment.
Warnings and Precautions
Before Starting Treatment with Oxaliplatin Kabi, Tell Your Doctor, Pharmacist, or Nurse If:
You Have Ever Had an Allergic Reaction to Platinum-Containing Medicines Such as Carboplatin or Cisplatin. An Allergic Reaction May Occur During Infusion of Oxaliplatin;
You Have Mild or Moderate Kidney Impairment;
You Have Liver Impairment or Abnormal Liver Function Tests;
You Have or Have Had Heart Problems, Such as Abnormal Heart Rhythms (So-Called QT Interval Prolongation), Irregular Heartbeats, or a Family History of Heart Problems;
You Have Recently Received or Plan to Receive Any Vaccines. You Should Not Receive "Live" or "Attenuated" Vaccines, Such as the Yellow Fever Vaccine, While Being Treated with Oxaliplatin;
If Any of the Following Apply to You, Tell Your Doctor Immediately. Your Doctor May Decide to Start Appropriate Treatment and May Reduce the Dose of Oxaliplatin Kabi or Delay or Stop Treatment.
If You Experience Unpleasant Sensations in the Throat (Especially When Swallowing) and Shortness of Breath, Tell Your Doctor;
If You Experience Nerve Damage in Your Hands or Feet, Such as Numbness or Tingling, or Decreased Sensation in Your Hands or Feet, Tell Your Doctor;
If You Experience Headache, Mental Changes, Seizures, and Vision Changes from Blurred Vision to Loss of Vision, Tell Your Doctor;
If You Experience Nausea or Vomiting, Tell Your Doctor;
If You Experience Severe Diarrhea, Tell Your Doctor;
If You Experience Mouth Ulcers or Mouth Sores (Mucositis), Tell Your Doctor;
If You Experience Diarrhea or a Decreased Number of White Blood Cells or Platelets, Tell Your Doctor. Your Doctor May Reduce the Dose of Oxaliplatin Kabi or Delay Administration;
If You Experience Unexplained Respiratory Symptoms, Such as Cough or Difficulty Breathing, Tell Your Doctor. Your Doctor May Decide to Stop Using Oxaliplatin Kabi;
If You Experience Extreme Fatigue, Shortness of Breath, or Kidney Disease with Decreased Urine Output or No Urine Output (Signs of Acute Kidney Failure), Tell Your Doctor;
If You Have a Fever (38°C or Higher) or Chills, Which May Indicate an Infection, Tell Your Doctor Immediately Due to the Risk of Developing a Blood Infection;
If You Have a Fever >38°C, Tell Your Doctor. Your Doctor May Check for a Decrease in the Number of White Blood Cells;
If You Experience Unexpected Bleeding or Bruising (Disseminated Intravascular Coagulation), Tell Your Doctor, as This May Be a Sign of Blood Clots in Small Blood Vessels;
If You Fainted (Lost Consciousness) or Have an Irregular Heartbeat, Tell Your Doctor Immediately, as This May Be a Sign of Severe Heart Problems;
If You Experience Muscle Pain and Have Swelling Along with Weakness, Fever, or Brownish-Red Urine, Tell Your Doctor, as This May Be a Sign of Muscle Damage (Rhabdomyolysis), Which Can Lead to Kidney Damage or Other Complications;
If You Experience Abdominal Pain, Nausea, Vomiting Blood, or Coffee Ground-Like Particles in Vomit, or Very Dark (Tarry) Stools, Which May Indicate a Stomach or Intestinal Ulcer (With Possible Bleeding or Perforation), Tell Your Doctor;
If You Experience Abdominal Pain, Bloody Diarrhea, and Nausea and Vomiting, Which May Be Caused by Reduced Blood Flow to the Intestinal Wall (Intestinal Ischemia), Tell Your Doctor.
Oxaliplatin Kabi and Other Medicines
Tell Your Doctor or Pharmacist About All Medicines You Are Taking or Have Recently Taken, as Well as Any Medicines You Plan to Take.
Pregnancy, Breast-Feeding, and Fertility
Pregnancy
Do Not Become Pregnant During Treatment and Use Effective Contraception. It Is Also Recommended to Use Contraception for 15 Months After Treatment Ends.
Men Are Advised Not to Plan to Father a Child During Treatment and for Up to 12 Months After Treatment Ends and to Use Contraceptive Measures During This Time.
If You Are Pregnant or Plan to Become Pregnant, It Is Very Important to Discuss This with Your Doctor Before Starting Treatment.
If You Become Pregnant During Treatment, Tell Your Doctor Immediately.
Breast-Feeding
Do Not Breast-Feed During Treatment with Oxaliplatin.
Fertility
Oxaliplatin May Have an Irreversible Effect on Fertility. Before Starting Treatment, Men Should Seek Advice on the Possibility of Sperm Preservation.
Women Planning to Become Pregnant After Oxaliplatin Treatment Should Consult a Genetic Counselor.
Before Taking Any Medicine, Consult Your Doctor or Pharmacist.
Driving and Using Machines
Treatment with Oxaliplatin May Increase the Risk of Dizziness, Nausea, and Vomiting, as Well as Other Neurological Symptoms That May Affect Your Gait and Balance.
If These Symptoms Occur, Do Not Drive or Operate Machines.
If You Experience Vision Changes During Treatment with Oxaliplatin Kabi, Do Not Drive, Operate Machines, or Perform Hazardous Activities.
3. How to Use Oxaliplatin Kabi
Oxaliplatin Kabi Is Only for Use in Adult Patients.
For Single Use Only.
Dosage
The Dose of Oxaliplatin Is Calculated Based on Body Surface Area. It Is Calculated Based on the Patient's Height and Weight.
The Usual Dose for Adult Patients Is 85 Mg/M² of Body Surface Area.
The Dose You Receive Will Also Depend on Your Blood Test Results and Whether You Have Experienced Any Side Effects from Oxaliplatin.
Method and Route of Administration
Oxaliplatin Is Prescribed by a Doctor Specializing in Cancer Treatment. You Will Be Treated by Medical Staff Who Will Prepare the Required Dose of Oxaliplatin.
Oxaliplatin Is Given as a Slow Injection into One of Your Veins (Intravenous Infusion) Over 2 to 6 Hours.
Oxaliplatin Kabi Is Given at the Same Time as Folinic Acid and Before 5-Fluorouracil Infusion.
Frequency of Administration
You Will Usually Receive the Medicine in an Intravenous Infusion Every 2 Weeks.
Duration of Treatment
Your Doctor Will Decide on the Duration of Treatment. Treatment Will Last for a Maximum of 6 Months When Oxaliplatin Is Used After Complete Removal of the Tumor.
Use of a Higher Than Recommended Dose of Oxaliplatin Kabi
Since This Medicine Is Administered by Medical Staff, It Is Highly Unlikely That You Will Receive Too Little or Too Much of the Medicine. In Case of Overdose, Side Effects May Be Enhanced.
Your Doctor May Apply Appropriate Treatment for Side Effects.
If You Have Any Further Questions About the Use of This Medicine, Ask Your Doctor, Pharmacist, or Nurse.
4. Possible Side Effects
Like All Medicines, This Medicine Can Cause Side Effects, Although Not Everybody Gets Them.
If You Experience Any Side Effects, It Is Important to Inform Your Doctor Before the Next Treatment Cycle.
The Following Side Effects May Occur.
You Should Contact Your Doctor Immediately If You Experience Any of the Following Symptoms:
Signs of Allergy or Anaphylactic Reaction with Sudden Symptoms Such as Rash, Itching, or Hives, Difficulty Swallowing, Swelling of the Face, Lips, Tongue, or Other Parts of the Body, Shortness of Breath, Wheezing, or Difficulty Breathing, Extreme Fatigue (You May Feel Like You Are Going to Faint); In Most Cases, These Symptoms Occur During or Immediately After Infusion, but Delayed Allergic Reactions Have Also Been Observed Several Hours or Even Days After Infusion;
Unusual Bruising, Bleeding, or Signs of Infection, Such as Sore Throat and High Temperature;
Persistent or Severe Diarrhea or Vomiting;
Blood in Vomit or Coffee Ground-Like Particles in Vomit;
Mouth Ulcers or Mucositis (Pain in the Lips or Mouth);
Respiratory Symptoms Such as Dry or Wet Cough, Difficulty Breathing, or Wheezing, Which May Be Signs of a Severe Lung Disease That Can Be Life-Threatening;
A Group of Symptoms Such as Headache, Changes in Mental Status, Seizures, and Vision Changes from Blurred Vision to Loss of Vision (Symptoms of a Condition Called Reversible Posterior Leukoencephalopathy Syndrome, a Rare Neurological Disorder);
Signs of Stroke (Including Sudden Severe Headache, Confusion, Problems with Vision in One or Both Eyes, Numbness or Weakness of the Face, Arm, or Leg, Usually on One Side, Drooping of the Face, Problems with Walking, Dizziness, and Problems with Speech);
Extreme Fatigue with Decreased Red Blood Cells and Shortness of Breath (Hemolytic Anemia) with or Without Decreased Platelets and Kidney Disease with Decreased Urine Output or No Urine Output (Symptoms of Hemolytic Uremic Syndrome).
Other Known Side Effects of Oxaliplatin Include:
Very Common (May Affect More Than 1 in 10 People):
- Oxaliplatin Kabi Affects the Nerves (Peripheral Neuropathy). You May Experience Tingling and Numbness of the Fingers and Toes, in the Mouth or Throat, Sometimes Accompanied by Cramps. This Side Effect Is Often Triggered When You Are Exposed to Cold, for Example, When Opening the Refrigerator or Holding a Cold Drink. You May Also Experience Difficulty Performing Precise Tasks, Such as
Buttoning. Although These Symptoms Usually Disappear Completely, There Is a Possibility of Persistent Peripheral Neuropathy Symptoms After Treatment Ends.
Some Patients May Experience a Tingling Sensation Like an "Electric Shock" Running Along the Arms or Chest When the Neck Is Bent;
Oxaliplatin Kabi May Sometimes Cause Unpleasant Sensations in the Throat, Especially When Swallowing, as Well as a Feeling of Shortness of Breath. Such Sensations, If They Occur, Usually Appear During Infusion or Within a Few Hours After Its Completion and May Be Triggered by Exposure to Low Temperatures. They Are Unpleasant but Do Not Last Long and Disappear Without the Need for Any Treatment. Your Doctor May Decide to Change the Treatment;
Oxaliplatin Kabi May Cause Diarrhea, Mild Nausea, and Vomiting. Your Doctor Will Prescribe an Anti-Emetic Medicine That Is Usually Given Before Treatment and Can Be Taken After the Treatment Cycle;
Oxaliplatin Kabi Causes a Temporary Decrease in Blood Cells. A Decrease in Red Blood Cells May Cause Anemia, a Decrease in Platelets May Cause Unusual Bleeding or Bruising, and a Decrease in White Blood Cells May Increase the Risk of Infections. Before Starting Treatment and Before Each Subsequent Cycle, Your Doctor Will Order a Blood Test to Check If You Have Enough Blood Cells;
Discomfort at the Injection Site or in Its Vicinity During Infusion;
Fever, Chills, Mild or Moderate Fatigue, Body Aches;
Changes in Weight, Decreased or Loss of Appetite, Taste Disorders, Constipation;
Headache, Back Pain;
Nerve Swelling in the Muscles, Stiffness of the Neck, Abnormal Tongue Sensation That May Affect Speech, Mouth Ulcers, Mucositis (Ulcers in the Mouth or Lips);
Abdominal Pain;
Abnormal Bleeding, Including Nosebleeds;
Cough, Difficulty Breathing;
Allergic Reactions, Skin Rash That May Be Red and Itchy, Slight Hair Loss (Alopecia);
Changes in Blood Test Results, Including Those Indicating Abnormal Liver Function.
Common (May Affect Less Than 1 in 10 People):
Infection Due to a Decreased Number of White Blood Cells;
Severe Blood Infection with a Decreased Number of White Blood Cells (Febrile Neutropenia), Which May Be Life-Threatening;
A Decrease in the Number of White Blood Cells with Fever >38.3°C or Prolonged Fever >38°C for More Than an Hour (Neutropenia with Fever);
Indigestion and Heartburn, Hiccups, Flushing of the Face, Dizziness;
Excessive Sweating and Nail Disorders, Skin Peeling;
Chest Pain;
Lung Disorders and Common Cold;
Bone and Joint Pain;
Pain When Urinating and Kidney Changes, Changes in Urine Frequency, Dehydration;
Blood in Urine or Stool, Blood Vessel Swelling, Blood Clots in the Lungs;
High Blood Pressure;
Depression, Insomnia;
Conjunctivitis, Vision Changes;
Decreased Calcium Levels in the Blood;
Falls.
Uncommon (May Affect Less Than 1 in 100 People):
Severe Blood Infection (Sepsis), Which May Be Life-Threatening;
Intestinal Obstruction or Swelling;
Nervousness.
Rare (May Affect Less Than 1 in 1,000 People):
Hearing Loss;
Scarring and Thickening of the Lungs, with Difficulty Breathing, Which Can Be Life-Threatening (Interstitial Lung Disease);
Temporary, Short-Term Loss of Vision;
Unexplained Bleeding or Bruising Due to Blood Clots in Small Blood Vessels (Disseminated Intravascular Coagulation) - a Condition That Can Be Life-Threatening.
Very Rare (Less Than 1 in 10,000 People):
Blood or Coffee Ground-Like Particles in Vomit;
Kidney Disease with Decreased Urine Output or No Urine Output (Signs of Acute Kidney Failure);
Liver Blood Vessel Disease.
Frequency Not Known (Cannot Be Estimated from the Available Data):
Allergic Blood Vessel Inflammation (Vasculitis);
An Autoimmune Reaction That Decreases the Number of All Blood Cells (Autoimmune Pancytopenia), Pancytopenia;
Severe Blood Infection with Low Blood Pressure (Septic Shock), Which May Be Life-Threatening;
Seizures (Uncontrolled Body Shaking);
Laryngeal Spasm Causing Difficulty Breathing;
Extreme Fatigue with Decreased Red Blood Cells and Shortness of Breath (Hemolytic Anemia) with or Without Decreased Platelets and Kidney Disease with Decreased Urine Output or No Urine Output (Symptoms of Hemolytic Uremic Syndrome), Which May Be Life-Threatening;
Abnormal Heart Rhythm (So-Called QT Interval Prolongation), Which Can Be Seen on an Electrocardiogram (ECG) - a Condition That Can Be Life-Threatening;
Muscle Pain and Swelling Along with Weakness, Fever, or Brownish-Red Urine (Symptoms of Muscle Damage Called Rhabdomyolysis) - a Condition That Can Be Life-Threatening;
Abdominal Pain, Nausea, Vomiting Blood, or Coffee Ground-Like Particles in Vomit, or Very Dark (Tarry) Stools (Symptoms of Stomach or Intestinal Ulcer with Possible Bleeding or Perforation) - a Condition That Can Be Life-Threatening;
Decreased Blood Flow to the Intestinal Wall (Intestinal Ischemia) - a Condition That Can Be Life-Threatening;
Risk of Developing New Tumors. Cases of Leukemia - a Type of Blood Cancer - Have Been Reported in Patients Treated with Oxaliplatin in Combination with Certain Other Medicines. You Should Discuss with Your Doctor the Risk of Potentially Developing This Type of Tumor During Treatment with Oxaliplatin and Certain Other Medicines;
Heart Attack (Myocardial Infarction), Angina (Chest Pain or Discomfort);
Esophagitis (Inflammation of the Esophagus, the Tube Connecting the Mouth to the Stomach);
Non-Cancerous Abnormal Growths in the Liver (Focal Nodular Hyperplasia).
Reporting Side Effects
If You Experience Any Side Effects, Including Any Not Listed in This Leaflet, Tell Your Doctor, Pharmacist, or Nurse. You Can Also Report Side Effects Directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
You Can Also Report Side Effects to the Marketing Authorization Holder.
By Reporting Side Effects, You Can Help Provide More Information on the Safety of This Medicine.
5. How to Store Oxaliplatin Kabi
Keep This Medicine Out of the Sight and Reach of Children.
Do Not Use This Medicine After the Expiration Date Stated on the Label and Carton After "EXP". The Expiration Date Refers to the Last Day of the Month.
Store the Vial in the Outer Carton to Protect from Light. Store Below 25°C. Do Not Freeze.
Do Not Use This Medicine If the Solution Is Not Clear and Visible Particles Are Present.
Medicines Should Not Be Disposed of via Wastewater or Household Waste. Ask Your Pharmacist How to Dispose of Medicines No Longer Required. This Will Help Protect the Environment.
6. Contents of the Pack and Other Information
What Oxaliplatin Kabi Contains:
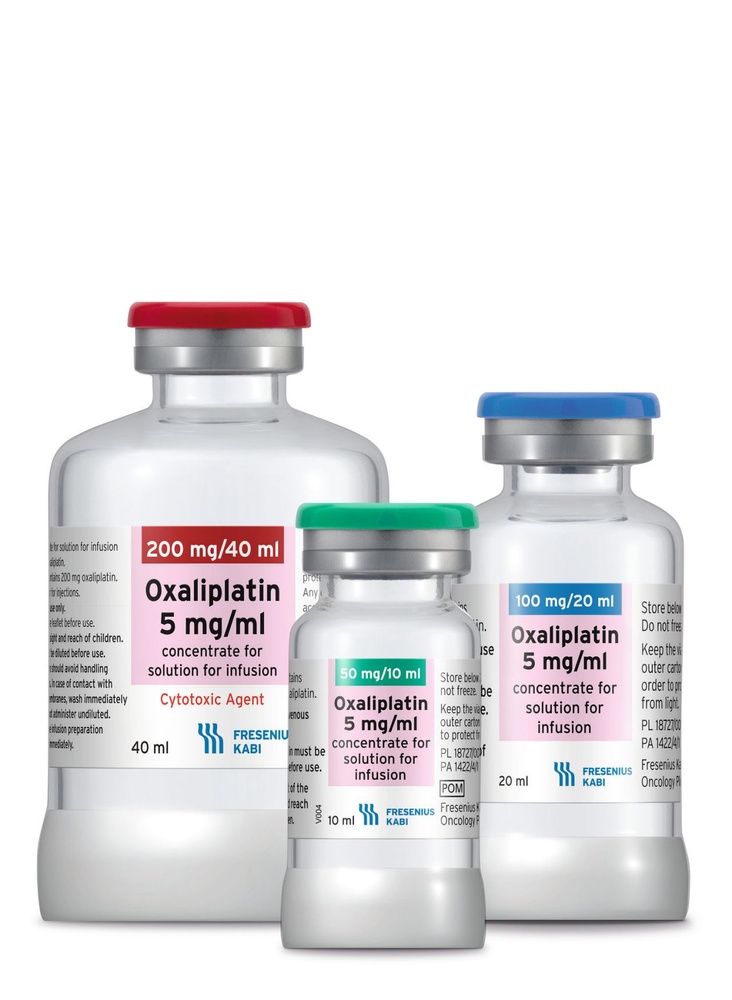
- The Active Substance Is Oxaliplatin. 1 Ml of the Concentrate for Solution for Infusion Contains 5 Mg of Oxaliplatin. 10 Ml of the Concentrate for Solution for Infusion Contains 50 Mg of Oxaliplatin. 20 Ml of the Concentrate for Solution for Infusion Contains 100 Mg of Oxaliplatin. 40 Ml of the Concentrate for Solution for Infusion Contains 200 Mg of Oxaliplatin.
- The Other Ingredients Are: Water for Injections.
What Oxaliplatin Kabi Looks Like and Contents of the Pack
This Medicine Is a Concentrate for Solution for Infusion. It Is a Clear, Colorless Solution Without Visible Particles.
One Vial Contains 50 Mg, 100 Mg, or 200 Mg of Oxaliplatin. 10 Ml, 20 Ml, or 40 Ml of the Concentrate Is in a Vial (Made of Colorless Glass Type I) with a Rubber Stopper Made of Chlorobutyl or Bromobutyl Rubber, with an Aluminum Seal and a Plastic Cap of the "Flip-Off" Type. Each Vial May Be Wrapped in Foil and a Plastic Container.
The Vials Are in a Carton.
Not All Pack Sizes May Be Marketed.
Marketing Authorization Holder
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
Manufacturer
Fresenius Kabi Deutschland GmbH
Pfingstweide 53
61169 Friedberg
Germany
For More Detailed Information, Please Contact the Marketing Authorization Holder:
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
Phone: +48 22 345 67 89
This Medicine Is Authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) Under the Following Names:
Czech Republic
Oxaliplatin Kabi
Denmark
Oxaliplatin "Fresenius Kabi", Concentrate for Solution for Infusion, Solution
Spain
Oxaliplatino Kabi 5 Mg/Ml Concentrate for Solution for Infusion EFG
Netherlands
Oxaliplatine Fresenius Kabi 5 Mg/Ml Concentrate for Solution for Infusion
Ireland
Oxaliplatin 5 Mg/Ml Concentrate for Solution for Infusion
Germany
Oxaliplatin Kabi 5 Mg/Ml Concentrate for Solution for Infusion
Norway
Oxaliplatin Fresenius Kabi 5 Mg/Ml Concentrate for Solution for Infusion
Poland
Oxaliplatin Kabi
Portugal
Oxaliplatina Kabi 5 Mg/Ml Concentrate for Solution for Infusion
Slovakia
Oxaliplatin Kabi 5 Mg/Ml Solution for Infusion Concentrate
Hungary
Oxaliplatin Kabi 5 Mg/Ml Concentrate for Solution for Infusion
Italy
Oxaliplatino Kabi
United Kingdom (Northern Ireland) Oxaliplatin 5 Mg/Ml Concentrate for Solution for Infusion
Date of Last Revision of the Leaflet:28.06.2024
---------------------------------------------------------------------------------------------------------------------------
Information Intended for Healthcare Professionals Only:
SPECIAL PRECAUTIONS FOR DISPOSAL AND PREPARATION OF THE MEDICINE
FOR ADMINISTRATION
As with Other Potentially Toxic Substances, Special Precautions Should Be Taken When Handling and Preparing Oxaliplatin Solutions.
Instructions for Use
The Use of Cytotoxic Medicines Requires Special Precautions to Ensure the Safety of the Person Preparing Them and Their Environment.
Preparation of Solutions for Injection of Cytotoxic Medicines Must Be Carried Out by Trained, Specialist Personnel with Knowledge of the Medicines Used, in Conditions That Guarantee the Integrity of the Medicine, Environmental Protection, and in Particular, the Protection of the Personnel Preparing the Medicine, in Accordance with Hospital Policy. Preparation of the Medicine Can Only Be Carried Out in a Designated Area. Smoking, Eating, or Drinking in This Area Is Prohibited.
Personnel Preparing Cytotoxic Medicines Must Be Equipped with Appropriate Personal Protective Equipment, Including Long-Sleeved Gowns, Protective Masks, Protective Head Covers, Protective Eyewear, Sterile Single-Use Gloves, Appropriate Protective Covers for the Work Area, Containers, and Waste Bags.
Care Should Be Taken When Handling Secretions or Vomit.
Pregnant Women Should Be Warned to Avoid Contact with Cytotoxic Medicines.
Damaged Packaging Should Be Treated with the Same Precautions as Contaminated Waste. Contaminated Waste Should Be Incinerated After Being Placed in Appropriately Labeled, Rigid Containers. See Below "Disposal of Unused Material".
In Case of Contact of the Oxaliplatin Concentrate or Infusion Solution with the Skin, the Area Should Be Rinsed Immediately with Plenty of Water.
In Case of Contact of the Oxaliplatin Concentrate or Infusion Solution with the Mucous Membranes, the Area Should Be Rinsed Immediately with Plenty of Water.
Special Precautions for Administration of the Medicine
- DO NOT Use Injection Equipment Containing Aluminum.
- DO NOT Administer Undiluted Solutions.
- For Dilution, Only Use 5% Glucose Solution for Infusion. DO NOT Dilute the Infusion Solution with Sodium Chloride Solution or Solutions Containing Chlorides.
- DO NOT Mix with Other Medicines in the Same Infusion Bag and DO NOT Administer Simultaneously in the Same Infusion Set.
- DO NOT Mix with Medicines or Solutions with an Alkaline pH, in Particular with 5-Fluorouracil and Folinic Acid Products Containing Tromethamine as an Excipient and Tromethamine Salts of Other Active Substances. Medicines or Solutions with an Alkaline pH Negatively Affect the Stability of Oxaliplatin.
Instructions for Use with Folinic Acid (FA) (as Folinic Acid or Disodium Folate)
Oxaliplatin at a Dose of 85 Mg/M² of Body Surface Area Administered in an Intravenous Infusion of 250 Ml to 500 Ml of 5% Glucose Solution Should Be Given at the Same Time as Folinic Acid Infusion in 5% Glucose Solution, Over 2 to 6 Hours, Using a Y-Connector Placed Immediately Before the Infusion Site. The Two Medicines Should Not Be Mixed in the Same Infusion Bag. Folinic Acid Should Not Contain Tromethamine as an Excipient and Should Be Diluted Only with Isotonic 5% Glucose Solution; Never Use Alkaline Solutions, Sodium Chloride Solution, or Solutions Containing Chlorides for Dilution.
Instructions for Use with 5-Fluorouracil (5-FU)
Oxaliplatin Should Always Be Administered Before Fluoropyrimidines, Such as 5-Fluorouracil.
After Administering Oxaliplatin, the Infusion Set Should Be Flushed, and Then 5-Fluorouracil Should Be Administered.
For Further Information on Medicines Used in Combination with Oxaliplatin, See the Relevant Summary of Product Characteristics.
USE ONLY the Recommended Diluents (See Below).
ANY Concentrate That Shows Signs of Precipitation Should NOT Be Used and Should Be Disposed of in Accordance with the Requirements for Disposal of Hazardous Waste (See Below).
Concentrate for Solution for Infusion
Before Use, the Solution Should Be Inspected. Only Clear Solutions Without Visible Particles Should Be Used. The Medicine Is for Single Use Only. Any Unused Solution Should Be Disposed of.
Dilution of the Infusion Solution
Take the Required Amount of Concentrate from the Vial(s) and Dilute with 250 Ml to 500 Ml of 5% Glucose Solution to Achieve an Oxaliplatin Concentration Between 0.2 Mg/Ml and 0.7 Mg/Ml. The Range of Concentrations for Which the Physico-Chemical Stability of Oxaliplatin Has Been Demonstrated Is from 0.2 Mg/Ml to 2.0 Mg/Ml.
Administer as an Intravenous Infusion.
After Dilution in 5% Glucose Solution, the Chemical and Physical Stability of the Solution Has Been Demonstrated for 24 Hours at Room Temperature (15°C to 25°C) and Under Refrigeration (2°C to 8°C).
From a Microbiological Point of View, the Infusion Solution Should Be Used Immediately.
If the Product Is Not Used Immediately, the Responsibility for the Storage Conditions and the Storage Time Before Use Lies with the User. The Storage Time Should Not Normally Be Longer Than 24 Hours at 2°C to 8°C, Unless the Solution Has Been Diluted Under Controlled and Validated Aseptic Conditions.
Before Use, the Solution Should Be Inspected. Only Clear Solutions Without Visible Particles Should Be Used. The Medicine Is for Single Use Only. Any Unused Solution Should Be Disposed of.
NEVER Use Sodium Chloride Solution or Solutions Containing Chlorides for Dilution.
Infusion
Administration of Oxaliplatin Does Not Require Prior Excessive Hydration of the Patient.
Oxaliplatin Diluted in 250 Ml to 500 Ml of 5% Glucose Solution to Achieve a Concentration of at Least 0.2 Mg/Ml Should Be Administered into a Peripheral or Central Vein Over 2 to 6 Hours.
When Oxaliplatin Is Administered with 5-Fluorouracil, the Oxaliplatin Infusion Should Precede the Administration of 5-Fluorouracil.
Disposal of Unused Material
Any Unused Medicine or Waste Should Be Disposed of in Accordance with Local Requirements.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterFresenius Kabi Deutschland GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Oxaliplatin KabiDosage form: Concentrate, 5 mg/mlActive substance: oxaliplatinPrescription not requiredDosage form: Concentrate, 5 mg/mlActive substance: oxaliplatinPrescription requiredDosage form: Concentrate, 5 mg/mlActive substance: oxaliplatinPrescription not required
Alternatives to Oxaliplatin Kabi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oxaliplatin Kabi in Spain
Alternative to Oxaliplatin Kabi in Ukraine
Online doctors for Oxaliplatin Kabi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oxaliplatin Kabi – subject to medical assessment and local rules.