
Ondansetron Kalceks
Ask a doctor about a prescription for Ondansetron Kalceks

How to use Ondansetron Kalceks
Leaflet attached to the packaging: information for the user
ONDANSETRON KALCEKS, 2 mg/ml, solution for injection/infusion
Ondansetron
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- In case of any doubts, consult a doctor, nurse, or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, nurse, or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is ONDANSETRON KALCEKS and what is it used for
- 2. Important information before using ONDANSETRON KALCEKS
- 3. How to use ONDANSETRON KALCEKS
- 4. Possible side effects
- 5. How to store ONDANSETRON KALCEKS
- 6. Contents of the packaging and other information
1. What is ONDANSETRON KALCEKS and what is it used for
ONDANSETRON KALCEKS contains the active substance ondansetron. It belongs to a group of antiemetic medicines that relieve nausea and vomiting.
Adults
Ondansetron is used to prevent and treat nausea and vomiting caused by chemotherapy and radiotherapy, and to prevent and treat nausea and vomiting after surgical operations.
Children and adolescents
Ondansetron is used to prevent and treat nausea and vomiting caused by chemotherapy in children over 6 months of age and in adolescents.
Ondansetron is used to prevent and treat nausea and vomiting after surgical operations in children over 1 month of age and in adolescents.
2. Important information before using ONDANSETRON KALCEKS
When not to use ONDANSETRON KALCEKS:
The patient will not receive ONDANSETRON KALCEKS if any of the above apply.
If the patient is unsure whether any of the above apply, they should talk to their doctor or nurse before using this medicine.
Warnings and precautions
Before starting treatment with ONDANSETRON KALCEKS, the patient should discuss it with their doctor or nurse if:
- the patient has symptoms of an allergic reaction, such as itching, difficulty breathing, or swelling of the face, lips, throat, or tongue;
- the patient has ever had an allergy to other antiemetic medicines (e.g., granisetron or palonosetron);
- the patient has heart problems; there may be a temporary change in the electrocardiogram (ECG);
- the patient is taking medicines for heart rhythm disorders (antiarrhythmic medicines) or medicines that lower blood pressure and heart rate at rest (beta-blockers);
- the patient has constipation or intestinal disease that may lead to constipation;
- the patient has liver problems or is taking any medicines that may be harmful to the liver (hepatotoxic medicines used in chemotherapy). In such cases, liver function will be closely monitored, especially in children and adolescents;
- a blood test has been performed to check liver function (ondansetron may affect the results);
- the patient has problems with mineral salt levels in the blood, such as potassium and magnesium;
- the patient is planning to have a tonsillectomy. In this case, the patient must be closely monitored.
If the patient is unsure whether any of the above apply, they should talk to their doctor or nurse before using this medicine.
ONDANSETRON KALCEKS and other medicines
The patient should tell their doctor or nurse about all medicines they are taking, have recently taken, or might take.
In particular, the patient should tell their doctor or nurse if they are taking any of the following medicines:
- apomorphine (see "When not to use ONDANSETRON KALCEKS");
- carbamazepine or phenytoin (used to treat epilepsy);
- rifampicin (used to treat infections such as tuberculosis);
- tramadol (a pain reliever);
- medicines used to treat depression and/or anxiety disorders:
- medicines from the group of selective serotonin reuptake inhibitors (SSRIs), including fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram, and escitalopram;
- medicines from the group of serotonin and norepinephrine reuptake inhibitors (SNRIs), including venlafaxine and duloxetine.
When used with medicines for certain heart conditions, changes in the ECG image may occur. Concomitant use of heart-damaging medicines (e.g., anthracyclines such as doxorubicin, daunorubicin) or antibiotics (e.g., erythromycin), antifungal medicines (e.g., ketoconazole), antiarrhythmic medicines (e.g., amiodarone), and beta-blockers (e.g., atenolol or timolol) may increase the risk of heart rhythm disorders.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
Pregnancy
ONDANSETRON KALCEKS should not be used during the first trimester of pregnancy. This is because ONDANSETRON KALCEKS may slightly increase the risk of the baby being born with a cleft lip and/or palate (a hole or gap in the upper lip and/or roof of the mouth).
If the patient is pregnant, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Women of childbearing age
Women of childbearing age may be advised to use effective contraception.
Breastfeeding
Breastfeeding should be stopped before starting treatment with ondansetron.
Fertility
Ondansetron has no effect on fertility.
Driving and using machines
Ondansetron has no or negligible influence on the ability to drive and use machines.
ONDANSETRON KALCEKS contains sodium
This medicine contains 3.52 mg of sodium (the main component of common salt) per ml of solution. This corresponds to 0.18% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to use ONDANSETRON KALCEKS
Ondansetron is given by a doctor or nurse as a slow injection or infusion into a vein or as an intramuscular injection.
Ondansetron is also available in other pharmaceutical forms suitable for rectal or oral administration, allowing for individualized dosing.
However, ONDANSETRON KALCEKS is intended for intravenous or intramuscular use only.
Adults
Prevention of nausea and vomiting caused by chemotherapy or radiotherapy
- On the day of chemotherapy or radiotherapyOndansetron will be given directly before chemotherapy or radiotherapy. The usual dose for adults is 8 mg, given as a slow intravenous or intramuscular injection, or slow intravenous infusion.
- In subsequent daysAfter initial treatment, the doctor may recommend that ondansetron be taken orally or rectally. If necessary, follow the instructions on the relevant packaging leaflet. Always take ondansetron as directed by the doctor.
If necessary, the dose can be increased to 32 mg per day.
Prevention and treatment of nausea and vomiting after surgical operations
The usual dose for adults is 4 mg given as a slow intravenous or intramuscular injection.
Children and adolescents
Prevention of nausea and vomiting caused by chemotherapy in children over 6 months of age and in adolescents
In children, the medicine is given slowly into a vein (intravenously) directly before chemotherapy (recommended dose: 5 mg/m or 0.15 mg/kg). The intravenous dose should not exceed 8 mg. Oral administration can be started 12 hours later. This treatment can be continued for up to 5 days after chemotherapy.
The oral dose is calculated based on body weight or body surface area. The total daily dose should not exceed the adult dose of 32 mg.
Prevention and treatment of nausea and vomiting after surgical operations in children over 1 month of age and in adolescents
In children, the dose is calculated based on body weight or body surface area. The total daily dose should not exceed the adult dose of 32 mg. The dose is given as a slow intravenous injection before, during, or after induction of anesthesia.
Elderly patients (over 65 years of age)
Ondansetron is well tolerated in elderly patients.
Nausea and vomiting caused by chemotherapy and radiotherapy
In patients over 65 years of age, all intravenous doses should be diluted and given as an infusion lasting at least 15 minutes. If multiple doses are necessary, they should be given at intervals of at least 4 hours.
In patients between 65 and 74 years of age, the initial dose is 8 mg or 16 mg. In patients over 75 years of age, the initial dose should not exceed 8 mg.
Prevention and treatment of nausea and vomiting after surgical operations
Data on use in elderly patients are limited.
Patients with liver function disorders
In patients with moderate or severe liver function disorders, the total daily dose should not exceed 8 mg.
Patients with kidney function disorders
No dose adjustment, frequency, or route of administration is required.
Use of a higher than recommended dose of ONDANSETRON KALCEKS
The doctor or nurse will give the patient an injection of ONDANSETRON KALCEKS, so it is unlikely that the patient will receive too much of the medicine. If it is thought that the patient has received too much of the medicine or has missed a dose, they should tell their doctor or nurse.
The following symptoms may occur: vision disturbances, severe constipation, low blood pressure, and slow heart rate.
If the patient has any further questions about the use of this medicine, they should ask their doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects in children and adolescents are similar to those in adults.
Severe allergic reactions.These occur rarely in people taking ondansetron. Symptoms include:
- A raised, itchy rash (hives)
- Swelling, sometimes of the face or lips (angioedema) with difficulty breathing
- Short-term loss of consciousness
If the patient experiences any of the above symptoms, they should contact their doctor immediately. They should stop using the medicine.
Very common side effects(may affect more than 1 in 10 people)
- Headache
Common side effects(may affect up to 1 in 10 people)
- Feeling of warmth or redness of the skin
- Constipation
- Redness
- Irritation at the injection site (after intravenous injection)
Uncommon side effects(may affect up to 1 in 100 people)
- Seizures
- Involuntary movements or muscle twitching
- Irregular or slow heartbeat
- Chest pain
- Low blood pressure
- Hiccup
- Increased liver enzyme activity
Rare side effects(may affect up to 1 in 1,000 people)
- Heart rhythm disorders (which can sometimes cause sudden loss of consciousness)
- Dizziness
- Transient blurred vision or vision disturbances
Very rare side effects(may affect up to 1 in 10,000 people)
- Widespread rash with blisters and skin peeling, covering a large area of the body (toxic epidermal necrolysis)
- Transient blindness
Frequency not known (cannot be estimated from the available data)
- Dry mouth
- Myocardial ischemia (symptoms include sudden chest pain or pressure in the chest)
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store ONDANSETRON KALCEKS
The medicine should be stored out of the sight and reach of children.
There are no special storage temperature requirements for the medicine.
Ampoules should be kept in the outer packaging to protect them from light.
After opening the ampoule
The medicine should be used immediately after opening.
Shelf life after dilution
Chemical and physical stability during use has been demonstrated for 7 days at 25°C and 2-8°C.
From a microbiological point of view, the diluted solution should be used immediately. If the solution is not used immediately, the user is responsible for the storage conditions and duration before use, which should not exceed 24 hours at 2-8°C, provided that the dilution has been made in controlled and validated aseptic conditions.
Do not use this medicine after the expiry date stated on the ampoule label after "EXP" and on the carton after "Expiry date (EXP)". The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer need. This will help protect the environment.
6. Contents of the packaging and other information
What ONDANSETRON KALCEKS contains
- The active substance is ondansetron. Each ml of solution contains ondansetron hydrochloride dihydrate equivalent to 2 mg of ondansetron. Each 2 ml ampoule contains ondansetron hydrochloride dihydrate equivalent to 4 mg of ondansetron. Each 4 ml ampoule contains ondansetron hydrochloride dihydrate equivalent to 8 mg of ondansetron.
- The other ingredients are sodium chloride, citric acid monohydrate, sodium citrate dihydrate, and water for injections.
What ONDANSETRON KALCEKS looks like and contents of the pack
Clear, colorless solution, free from visible particles.
2 ml or 4 ml solution in colorless glass type I ampoules with a colored ring.
Ampoules are packaged in a blister pack. The blister pack is packaged in a cardboard box.
Pack sizes:
5, 10, or 25 ampoules
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
AS KALCEKS
Krustpils iela 71E
LV-1057 Rīga
Latvia
Phone: +371 67083320
Email: [email protected]
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Latvia
Ondansetron Kalceks 2 mg/ml šķīdums injekcijām/infūzijām
Austria, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, Germany, Hungary, Iceland, Lithuania, Norway, Slovakia, Sweden:
Ondansetron Kalceks
Belgium
Ondansetron Kalceks 2 mg/ml, solution injectable/pour perfusion
Ondansetron Kalceks 2 mg/ml, oplossing voor injectie/infusie
Ondansetron Kalceks 2 mg/ml, Injektions-/Infusionslösung
Greece
ONDANSETRON/KALCEKS
Ireland, United Kingdom (Northern Ireland): Ondansetron 2 mg/ml solution for injection/infusion
Italy
Ondansetrone Kalceks
Netherlands
Ondansetron Kalceks 2 mg/ml, oplossing voor injectie/infusie
Poland
ONDANSETRON KALCEKS
Romania
Ondansetron Kalceks 2 mg/ml soluţie injectabilă/perfuzabilă
Slovenia
Ondansetron Kalceks 2 mg/ml raztopina za injiciranje/infundiranje
Spain
Ondansetron Kalceks 2 mg/ml soluciόn inyectable y para perfusiόn
Date of last revision of the leaflet: 02/2022
------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
More information about this medicine can be found in the Summary of Product Characteristics (SmPC).
Overdose
Symptoms and signs
Experience with ondansetron overdose is limited, but in the event of accidental overdose, the following symptoms of poisoning can be expected: vision disturbances, severe constipation, hypotension, and a vasovagal episode with transient second-degree atrioventricular block. In all cases, symptoms resolved completely. Ondansetron prolongs the QT interval in a dose-dependent manner.
Children and adolescents
After unintentional oral overdose of ondansetron (exceeding the estimated intake of 4 mg/kg) in infants and children aged 12 months to 2 years, pediatric cases indicating serotonin syndrome have been reported.
Management
There is no specific antidote for ondansetron. In case of suspected overdose, appropriate symptomatic and supportive treatment should be used. ECG monitoring is recommended. Further treatment should be in line with clinical indications or the recommendations of the national poison control center, if possible.
It is not recommended to use ipecacuanha for the treatment of overdose, as it is unlikely that patients will respond due to the antiemetic effect of ondansetron itself.
Incompatibilities
ONDANSETRON KALCEKS solution for injection/infusion should not be administered in the same syringe or infusion set as any other medicine.
This medicinal product should not be mixed with other medicinal products except those mentioned below.
Special precautions for disposal and preparation of the medicinal product for administration
For single use only.
The medicinal product should be inspected visually before use. The medicinal product should not be used if there are any signs of deterioration (e.g., particles or color change).
ONDANSETRON KALCEKS should not be sterilized in an autoclave.
The product may be diluted with the following intravenous infusion solutions:
- 9 mg/ml (0.9%) sodium chloride solution;
- 50 mg/ml (5%) glucose solution;
- 100 mg/ml (10%) mannitol solution;
- Ringer's solution;
- 3 mg/ml (0.3%) potassium chloride and 9 mg/ml (0.9%) sodium chloride;
- 3 mg/ml (0.3%) potassium chloride and 50 mg/ml (5%) glucose;
- Ringer's solution with lactate.
It has been demonstrated that ONDANSETRON KALCEKS is compatible with polypropylene syringes (PP), glass type I bottles, polyethylene (PE) infusion bags, polyvinyl chloride (PVC) and ethylene-vinyl acetate (EVA) infusion bags, and PVC and PE infusion lines after dilution with the above-mentioned infusion solutions. It has been demonstrated that undiluted ONDANSETRON KALCEKS solution for injection/infusion is compatible with PP syringes.
Compatibility with other medicines
Ondansetron can be administered as an intravenous infusion (1 mg per hour). The following medicinal products can be administered through a Y-site into the ondansetron infusion line for ondansetron concentrations between 16 and 160 µg/ml (e.g., 8 mg/500 ml and 8 mg/50 ml):
- Cisplatin
- 5-Fluorouracil
- Carboplatin
- Etoposide
- Ceftazidime
- Cyclophosphamide
- Doxorubicin
- Dexamethasone
Instructions for opening the ampoule
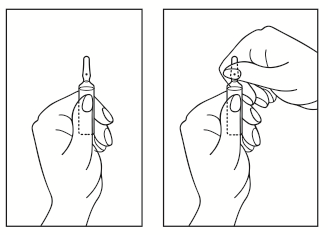
- 1) Turn the ampoule with the colored spot upwards. If there is a solution in the upper part of the ampoule, gently tap with your finger to move the entire solution to the lower part of the ampoule.
- 2) Use both hands to open the ampoule; holding the lower part of the ampoule in one hand, break the upper part of the ampoule in the direction away from the colored spot (see the picture below).
Any unused medicinal product or waste material should be disposed of in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAS Kalceks
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ondansetron KalceksDosage form: Tablets, 8 mgActive substance: ondansetronManufacturer: Egis Pharmaceuticals PLCPrescription requiredDosage form: Solution, 2 mg/mlActive substance: ondansetronPrescription requiredDosage form: Solution, 4 mgActive substance: ondansetronPrescription required
Alternatives to Ondansetron Kalceks in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ondansetron Kalceks in Spain
Alternative to Ondansetron Kalceks in Ukraine
Online doctors for Ondansetron Kalceks
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ondansetron Kalceks – subject to medical assessment and local rules.














