

Oculobrim

Ask a doctor about a prescription for Oculobrim

How to use Oculobrim
Package Leaflet: Information for the User
Oculobrim, 2 mg/ml, eye drops, solution
Brimonidine tartrate
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist. See section 4.
Contents of the package leaflet
- 1. What Oculobrim is and what it is used for
- 2. Important information before using Oculobrim
- 3. How to use Oculobrim
- 4. Possible side effects
- 5. How to store Oculobrim
- 6. Contents of the pack and other information
1. What Oculobrim is and what it is used for
Oculobrim contains the active substance brimonidine tartrate, which reduces pressure in the eye.
Oculobrim is used to reduce pressure in the eye.
It can be used alone, if eye drops with beta-adrenergic blockers are contraindicated, or together with other eye drops if a single medicine does not sufficiently reduce increased pressure in the eye, in the treatment of glaucoma with an open angle or intraocular hypertension.
2. Important information before using Oculobrim
When not to use Oculobrim
- if you are allergic to brimonidine or any of the other ingredients of this medicine (listed in section 6).
- in infants and young children (from birth to 2 years of age).
- if you are taking monoamine oxidase inhibitors (MAOIs) or certain antidepressants. You must inform your doctor if you are taking any antidepressant medication.
- if you are breastfeeding.
Warnings and precautions
Before starting treatment with Oculobrim, discuss with your doctor or pharmacist:
- if you are using it in a child aged 2 to 12 years, as Oculobrim is not recommended for use in this age group,
- if you have or have had depression, mental impairment, reduced blood flow to the brain, heart problems, blood flow disorders in the limbs or blood pressure disorders,
- if you have or have had kidney or liver problems.
If any of the above points apply to you, consult your doctor before starting treatment with Oculobrim.
Children and adolescents
Do not use this medicine in children and adolescents under 12 years of age, as the safety and efficacy of Oculobrim in this age group have not been established. This is particularly important in children under 2 years of age.
Oculobrim and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have recently taken, as well as any medicines you plan to take.
Tell your doctor if you regularly consume alcohol or take any of the following medicines:
- analgesics, sedatives, opioids, barbiturates,
- anesthetics,
- cardiovascular or blood pressure-lowering medicines,
- medicines that may affect metabolism, such as chlorpromazine, methylphenidate, or reserpine,
- medicines that act on the same receptor as Oculobrim, such as isoproterenol or prazosin,
- monoamine oxidase inhibitors (MAOIs) and other antidepressants,
- medicines used for any disease, even if not related to eye disorders, that you are suffering from,
- or in case of a change in the dose of currently used medicines.
These medicines may affect treatment with Oculobrim.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Do not use Oculobrim during breastfeeding.
Driving and using machines
Oculobrim may cause blurred vision or other vision disturbances. These disturbances may seem stronger at night or in low light.
In some patients, Oculobrim may also cause drowsiness or fatigue.
If you experience any of these symptoms, do not drive or operate machinery until they have resolved.
Oculobrim contains a preservative called benzalkonium chloride
The preservative in Oculobrim, benzalkonium chloride, may cause eye irritation and discoloration of soft contact lenses. Therefore, avoid wearing soft contact lenses. If you wear contact lenses, remove them before instillation and wait 15 minutes after instillation of Oculobrim before putting them back on.
3. How to use Oculobrim
Always use this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
Adults (including the elderly):
The recommended dose is one drop into the affected eye(s) twice daily, approximately 12 hours apart.
Use in children under 12 years of age
Do not use Oculobrim in children under 2 years of age.
Oculobrim is not recommended for use in children (aged 2 to 12 years).
Method of administration
Oculobrim is an eye drop solution. Before instillation, always wash your hands. Your doctor has prescribed the number of drops to be instilled into the eye at each dose. If you are using Oculobrim with other eye drops, wait 15 minutes before instilling the second medicine.
Instill the eye drops as follows:
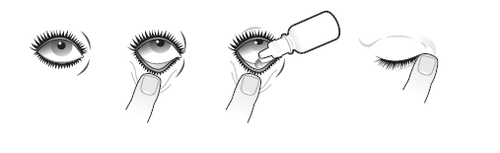
- 1. Tilt your head back and look up.
- 2. Gently pull down the lower eyelid to form a small pocket.
- 3. Turn the bottle upside down and squeeze it to administer one drop of the medicine into the eye.
- 4. With the eye closed, press the inner corner of the eye with your finger for 1 minute. Avoid touching the tip of the dropper to the eye or anything else. Immediately after use, replace the cap and tighten it.
Using more Oculobrim than prescribed
Adults
Side effects observed in patients who have instilled more drops than prescribed by the doctor are similar to the known side effects of Oculobrim.
In adults who have accidentally ingested Oculobrim, there has been a decrease in blood pressure, followed by an increase in blood pressure in some patients. In such a situation, contact your doctor immediately.
Children
Severe side effects have been reported in children who have accidentally ingested Oculobrim. The observed symptoms included drowsiness, limpness, decreased body temperature, paleness, and breathing difficulties. In such a situation, contact your doctor immediately.
Adults, adolescents, and children
In case of accidental ingestion or use of a higher dose of Oculobrim than prescribed, contact your doctor immediately.
Missing a dose of Oculobrim
If you miss a dose, use it as soon as you remember.
However, if it is almost time for the next dose, skip the missed dose and return to your regular dosing schedule.
Do not use a double dose to make up for a missed dose.
Stopping treatment with Oculobrim
The effective action of Oculobrim requires its daily use. Do not stop using Oculobrim unless your doctor advises you to do so.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Oculobrim can cause side effects, although not everybody gets them.
Some side effects may be serious. These include:
- fainting
- local irritation (inflammation and swelling of the eyelid, swelling of the cornea, sticky eyes, pain, and tearing)
- eye irritation (redness, burning, stinging, feeling of a foreign body in the eye, itching, follicles or white spots on the cornea)
- eye inflammation
- allergic reaction in the eye
- palpitations or changes in heart rate
- shortness of breath
- general allergic reactions
- skin reactions, including redness, swelling of the face, itching, rash, and vasodilation.
If you experience any of the above or other serious symptoms, contact your doctor or the nearest hospital emergency department for appropriate treatment.
The following side effects have been reported:
Eye disorders
Very common(may affect more than 1 in 10 people):
- eye irritation (redness, burning, stinging, feeling of a foreign body in the eye, itching, blisters or white spots on the cornea);
- blurred vision;
- allergic reaction in the eye.
Common(may affect up to 1 in 10 people):
- local irritation (inflammation and swelling of the eyelid, swelling of the cornea, sticky eyes, pain, and tearing);
- increased sensitivity to light;
- corneal defects and discoloration;
- dry eye;
- corneal clouding;
- vision disturbances;
- corneal inflammation.
Very rare(may affect up to 1 in 10,000 people):
- eye inflammation;
- pupil constriction.
Frequency not known(frequency cannot be estimated from the available data):
- eyelid itching.
General disorders
Very common(may affect more than 1 in 10 people):
- headache;
- dry mouth;
- fatigue/drowsiness.
Common(may affect up to 1 in 10 people):
- dizziness;
- cold-like symptoms;
- stomach and indigestion problems;
- abnormal taste;
- general weakness.
Uncommon(may affect up to 1 in 100 people):
- depression;
- palpitations or changes in heart rate;
- dry nose;
- general allergic reactions.
Rare(may affect up to 1 in 1,000 people):
- shortness of breath. Very rare(may affect up to 1 in 10,000 people):
- insomnia;
- fainting;
- high blood pressure;
- low blood pressure.
Frequency not known(frequency cannot be estimated from the available data):
- skin reactions, including redness, swelling of the face, itching, rash, and vasodilation.
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, e-mail: [email protected].
Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Oculobrim
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and bottle after "EXP".
The expiry date refers to the last day of that month.
The batch number (LOT) is printed on the packaging.
Discard the bottle after 28 days of first opening, even if there is still some solution left.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Oculobrim contains
The active substance is brimonidine tartrate.
1 ml of solution contains 2 mg of brimonidine tartrate, equivalent to 1.3 mg of brimonidine.
1 drop of solution contains 65.2 micrograms of brimonidine tartrate, equivalent to 43 micrograms of brimonidine.
The other ingredients are benzalkonium chloride, polyvinyl alcohol, sodium citrate, citric acid monohydrate, sodium chloride, purified water, hydrochloric acid, sodium hydroxide.
What Oculobrim looks like and contents of the pack
Oculobrim is a clear, greenish-yellow to light greenish-yellow solution for eye drops, supplied in a LDPE bottle with a dropper and an HDPE cap with a tamper-evident seal.
Each bottle contains 5 ml of eye drop solution.
Pack sizes: 1 x 5 ml or 3 x 5 ml
Not all pack sizes may be marketed.
Marketing authorization holder
Synoptis Pharma Sp. z o.o.
ul. Krakowiaków 65
02-255 Warsaw
Poland
Manufacturer
Jadran-Galenski Laboratorij d.d.
Svilno 20
51000 Rijeka
Croatia
Date of last revision of the package leaflet: November 2019
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OculobrimDosage form: Drops, 2 mg/mlActive substance: brimonidineManufacturer: Allergan Pharmaceuticals IrelandPrescription requiredDosage form: Drops, 2 mg/mlActive substance: brimonidinePrescription requiredDosage form: Drops, 2 mg/mlActive substance: brimonidineManufacturer: Rafarm S.A.Prescription required
Alternatives to Oculobrim in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oculobrim in Ukraine
Alternative to Oculobrim in Spain
Online doctors for Oculobrim
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oculobrim – subject to medical assessment and local rules.














