
Nicorette Cool Berri
Ask a doctor about a prescription for Nicorette Cool Berri

How to use Nicorette Cool Berri
Leaflet accompanying the packaging: patient information
Nicorette Cool Berry, 1 mg/dose, oral spray, solution
Nicotine
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as advised by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 6 months of using Nicorette Cool Berry you are still unable to stop smoking, consult a doctor.
Table of contents of the leaflet:
- 1. What is Nicorette Cool Berry and what is it used for
- 2. Important information before using Nicorette Cool Berry
- 3. How to use Nicorette Cool Berry
- 4. Possible side effects
- 5. How to store Nicorette Cool Berry
- 6. Contents of the pack and other information
1. What is Nicorette Cool Berry and what is it used for
Nicorette Cool Berry is used to help smokers quit smoking, either by quitting immediately or by gradually reducing smoking before quitting completely. This is a type of treatment called nicotine replacement therapy (NRT).
Nicorette Cool Berry alleviates withdrawal symptoms that occur after quitting smoking, including nicotine cravings. When nicotine from tobacco is suddenly stopped, various unpleasant feelings called withdrawal symptoms develop. By using Nicorette Cool Berry, you can prevent or reduce the severity of these unpleasant feelings and reduce the desire to light a cigarette. This is because a small amount of nicotine is continuously delivered to the body for a short time. Nicorette Cool Berry does not contain tar, carbon monoxide, and other toxins found in tobacco smoke.
To increase the chances of a successful quit attempt, it is also recommended to use counseling and psychological support.
2. Important information before using Nicorette Cool Berry
When not to use Nicorette Cool Berry
- If you are allergicto nicotine or any of the other ingredients of this medicine (listed in section 6).
- If you are under 18 years old.
- If you have never smoked.
Warnings and precautions
Before starting to use this medicine, discuss it with your doctor if you have any of the following conditions. You may be able to use Nicorette Cool Berry, but you must first consult your doctor if:
- you have recently (within the last 3 months) had a heart attackor stroke,
- you have chest pain(unstable angina) or symptoms of angina at rest,
- you have heart diseasethat affects the speed or regularity of your heartbeat,
- you have high blood pressurethat is not controlled by medication,
- you have ever had allergic reactions, such as swelling of the lips, face, and throat (angioedema) or itchy rash (hives); using NRT may occasionally provoke this type of reaction,
- you have severe or moderate liver disease,
- you have severe kidney disease,
- you have diabetes,
- you have hyperthyroidism,
- you have an adrenal gland tumor(pheochromocytoma),
- you have stomach or duodenal ulcers,
- you have esophagitis,
- you have had epilepsyor seizuresin the past.
Nicorette Cool Berry should not be used by non-smokers.
Children and adolescents
This medicine should not be used in children and adolescents.
Nicorette Cool Berry and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take, including those obtained without a prescription. This is especially important if you are taking medicines that contain:
- theophyllinefor asthma,
- tacrinefor Alzheimer's disease,
- clozapinefor schizophrenia,
- ropinirolefor Parkinson's disease.
Nicorette Cool Berry with food and drink
Do not eat or drink while using the oral spray.
Pregnancy, breastfeeding, and fertility
It is very important to stop smoking during pregnancy, as it can lead to fetal growth restriction and may cause miscarriage or stillbirth. It is best to try to quit smoking without using nicotine-containing medications. If this is not possible, using Nicorette Cool Berry can be started only after consulting the doctor managing the pregnancy, primary care physician, or a doctor specializing in smoking cessation.
Nicorette Cool Berry should not be taken during breastfeeding, as nicotine passes into breast milk and may affect the baby. If the doctor recommends using Nicorette Cool Berry, the spray should be used immediately after breastfeeding and no later than 2 hours before starting breastfeeding.
Smoking increases the risk of infertility in women and men. The effect of nicotine on fertility is unknown.
Driving and using machines
No effects on the ability to drive or use machines have been observed.
Nicorette Cool Berry contains propylene glycol (E 1520), ethanol, sodium, and butylhydroxytoluene (E 321)
Propylene glycol (E 1520)
The medicine contains 12 mg of propylene glycol in each dose.
Ethanol
This medicine contains approximately 7 mg of alcohol (ethanol) in each dose, which is equivalent to 97 mg/ml. The amount of alcohol in one dose of this medicine is equivalent to less than 2 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine will not have noticeable effects.
Sodium
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means the medicine is considered "sodium-free".
Butylhydroxytoluene (E 321)
Due to the presence of butylhydroxytoluene, Nicorette Cool Berry may cause local skin reactions (e.g., contact dermatitis) or eye irritation.
3. How to use Nicorette Cool Berry
This medicine should always be used exactly as described in this patient leaflet or as advised by a doctor or pharmacist. In case of doubt, consult a doctor or pharmacist.
Persons under 18 years oldshould not use Nicorette Cool Berry.
If after 6 weeks of treatment, it is not possible to reduce the number of doses used or the number of cigarettes smoked, or if the patient needs to use Nicorette Cool Berry for a period longer than 6 months, consult a doctor. Nicorette Cool Berry is usually used for 3 months, but not longer than 6 months.
The way to use Nicorette Cool Berry depends on whether the patient wants to quit smoking immediately or gradually by progressively reducing the number of cigarettes smoked before quitting completely.
Immediate quitting
The goal is to quit smoking immediately and use the spray to reduce nicotine cravings.
Do not use more than 2 doses at a time or 4 doses per hour for 16 hours. The maximum dose is 64 doses (applications) per 16 hours per day.
Stage 1:weeks 1-6
Use 1 or 2 doses instead of reaching for a cigarette or when experiencing nicotine cravings. First, use one dose, and if the craving does not disappear within a few minutes, use a second dose. If two doses are needed, they can be used at the same time next time. For most smokers, this means 1 or 2 doses every 30 minutes to 1 hour.
For example, if the average number of cigarettes smoked per day is 15, use 1-2 doses at least 15 times a day.
Stage 2:weeks 7-9
Start reducing the number of doses used per day.
By the end of week 9, the patient should be using HALF of the average number of doses used in stage 1.
Stage 3:weeks 10-12
Continue to reduce the number of doses used per day, so that by week 12, no more than 4 doses are used per day. When the number of doses per day is reduced to 2-4, stop using Nicorette Cool Berry.
Gradual quitting
This involves starting to gradually replace some cigarettes with Nicorette Cool Berry. Once this goal is achieved, the patient will completely stop smoking cigarettes and use the spray. Finally, the spray will also be stopped.
When experiencing a strong desire to smoke, use 1 or 2 doses of Nicorette Cool Berry instead of a cigarette to control nicotine cravings. The spray is used to replace cigarettes, so do not smoke after using the spray. Using the spray without reducing the number of cigarettes smoked can lead to nicotine overdose symptoms (see "Using more than the recommended dose of Nicorette Cool Berry"). Reduce the number of cigarettes smoked per day to the minimum and replace them with the spray. If after 6 weeks of treatment, it is not possible to reduce the number of cigarettes smoked per day, consult a doctor. The patient should quit smoking as soon as they feel ready, but no later than 12 weeks after starting treatment. After completely quitting smoking, gradually reduce the number of doses used per day. When the number of doses is reduced to 2-4 per day, stop using Nicorette Cool Berry.
Do not use more than 2 doses at a time or 4 doses per hour for 16 hours. The maximum dose is 64 doses (applications) per 16 hours per day.
After completing treatment, the desire to smoke may still be present. Do not discard any remaining medicine, as nicotine cravings can occur suddenly. If cravings occur, use one or two doses, depending on whether one dose provides improvement within a few minutes.
Follow the instructions below, guided by the diagrams:
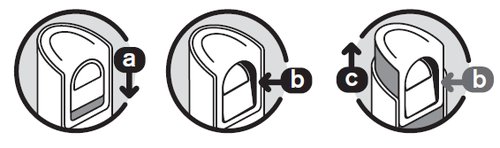
Unlocking the pump
Opening the dispenser
- 1. Using your thumb, slide the button ( a) down until it can be gentlypressed inward ( b). Do not press too hard.
- 2. Holding the button, slide it up ( c) to extend the pump. Then release the button.
Priming the dispenser
Before first using the oral spray, prime the pump by pointing the spray away from yourself, other adults, children, or pets. Press the top of the dispenser three times with your index finger until a fine mist appears. If the spray is not used for 2 days, repeat the priming procedure.
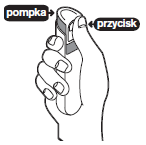
Press the top of the dispenser three times with your index finger until a fine mist appears.
Using the dispenser
- 3. Point the spray toward the open mouth, holding it as close to the mouth as possible.
- 4. Press the top of the dispenser firmly and spray one dose into the mouth, trying to avoid the lips. Do not inhale while spraying to avoid getting the spray into the throat. For optimal treatment results, do not swallow for a few seconds after spraying.
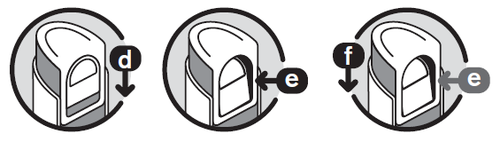
Locking the pump
Closing the dispenser
- 5. Slide the button down ( d) until it can be pressed inward ( e).
- 6. While pressing the button, slide the pump down ( f). Release the button. The dispenser is now closed. To use another dose, repeat the above steps.
Close the dispenser after each use to prevent children from using the spray or accidental release of the medicine.
Be careful not to direct the oral spray toward the eyes. If the spray gets into an eye, rinse it thoroughly with water.
Using more than the recommended dose of Nicorette Cool Berry
If you smoke while using Nicorette Cool Berry, nicotine overdose may occur.
If a child uses Nicorette Cool Berry or if you use more Nicorette Cool Berry than recommended, immediatelyconsult a doctor or go to the nearest hospital. Nicotine doses tolerated during treatment by adult smokers can cause severe poisoning and lead to deathin children.
Symptoms of overdose are nausea, vomiting, excessive salivation, abdominal pain, diarrhea, excessive sweating, headache, dizziness, hearing disturbances, and significant weakness.
After these symptoms, large doses may cause low blood pressure, weak and irregular heartbeat, breathing difficulties, exhaustion, vascular collapse, and generalized seizures.
In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Nicorette Cool Berry may cause the same side effects as other nicotine products.
These effects are generally dose-dependent.
Side effects related to quitting smoking (nicotine withdrawal)
Some of the side effects that occur when quitting smoking may be withdrawal symptoms caused by reducing the nicotine dose.
Possible side effects include:
- irritability, aggression, frustration, or impatience,
- feeling anxious, restless, or having difficulty concentrating,
- waking up at night or sleep disturbances,
- increased appetite or weight gain,
- feeling unwell,
- craving for cigarettes ( nicotine cravings),
- decreased heart rate,
- gum bleeding or mouth ulcers,
- dizziness or feeling empty-headed,
- cough, sore throat, stuffy nose, or runny nose,
- constipation.
Stop using Nicorette Cool Berry and consult a doctor immediately if you experience any of the following rare and serious side effects (symptoms of angioedema):
- swollen face, tongue, or throat,
- difficulty swallowing,
- hives and difficulty breathing.
Very common (may affect more than 1 in 10 people):
- hiccups (especially common),
- headache, nausea (nausea),
- throat irritation.
Common (may affect up to 1 in 10 people):
- local reactions, such as burning sensation, mouth irritation, changes in taste,
- dry mouth or increased salivation,
- discomfort in the mouth,
- abdominal pain or discomfort,
- nausea, vomiting, bloating, or diarrhea,
- feeling tired,
- allergic reactions (hypersensitivity),
- tingling,
- cough.
Uncommon (may affect up to 1 in 100 people):
- nose problems, such as congestion, sneezing,
- runny nose,
- wheezing (bronchospasm) or feeling more breathless than normal (dyspnea), tightness in the throat,
- redness of the skin or increased sweating,
- mouth reactions, such as tingling in the mouth, tongue inflammation, mouth ulcers, damage to the mouth mucosa, or change in voice, mouth pain and throat, belching, gum bleeding,
- palpitations (abnormal sensation of rapid or forceful heartbeat), rapid heartbeat, high blood pressure,
- skin rash and (or) itching (pruritus, hives),
- unusual dreams,
- discomfort and chest pain,
- weakness, feeling unwell.
Rare (may affect up to 1 in 1,000 people):
- difficulty swallowing, decreased sensation in the mouth,
- gag reflex.
Unknown (frequency cannot be estimated from the available data):
- blurred vision, increased tear production (lacrimation),
- dry throat, stomach discomfort, lip pain,
- heart rhythm disturbances,
- redness of the skin,
- allergic reactions, including swelling of the face and mouth (angioedema or anaphylaxis),
- seizures.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Nicorette Cool Berry
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the dispenser and outer packaging. The expiry date refers to the last day of the month.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment, especially the aquatic environment.
6. Contents of the pack and other information
What Nicorette Cool Berry contains
- The active substance is nicotine. One dose delivers 1 mg of nicotine.
- The other ingredients are: propylene glycol (E 1520), anhydrous ethanol, tromethamine, poloxamer 407, glycerol (E 422), sodium hydrocarbonate, levomenthol, red fruit flavor, cooling flavor, sucralose, acesulfame potassium, butylhydroxytoluene (E 321), hydrochloric acid (for pH adjustment), and purified water.
What Nicorette Cool Berry looks like and contents of the pack
Nicorette Cool Berry consists of a plastic bottle containing a solution in a dispenser with a mechanical pump. The dispenser is equipped with a child-resistant lock.
Each bottle contains 13.2 ml of solution, equivalent to 150 doses.
Nicorette Cool Berry is available in packs containing 1, 2, or 3 dispensers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
McNeil AB, Box 941, SE-251 09 Helsingborg, Sweden
Manufacturer:
McNeil AB, Norrbroplatsen 2, SE-251 09 Helsingborg, Sweden
Johnson & Johnson Consumer NV/SA, Michel De Braeystraat 52, 2000 Antwerp, Belgium
Johnson & Johnson GmbH, Johnson & Johnson Platz 2, Nordrhein-Westfalen, 41470 Neuss, Germany
For more information, contact:
email: [email protected]
This medicinal product is authorized in the Member States of the European
Economic Area under the following names:
Date of last revision of the leaflet: April 2025
| Sweden | Nicorette Fruktmint |
| Austria | Nicorette Fruit & Mint Spray |
| Belgium / Netherlands | Nicorette Fruit & Mint Mondspray |
| Bulgaria | Nicorette Berrymint |
| Croatia | Nicorette s okusom šumskog voća |
| Cyprus | Nicorette Quickspray Berry |
| Czech Republic | Nicorette Spray s příchutí lesního ovoce |
| Denmark | Nicorette QuickMist Cool Berry |
| Estonia | Nicorette Coolberry |
| Finland | Nicorette Berrymint |
| France | NICORETTESPRAY FRUITS ROUGES |
| Germany | Nicorette Fruit & Mint Spray |
| Greece | Nicorette Quickspray Berry |
| Hungary | Nicorette Berrymint |
| Iceland | Nicorette Quickmist Cool Berry |
| Ireland | Nicorette QuickMist Cool Berry |
| Italy | Nicorettequick |
| Latvia | Nicorette Coolberry |
| Luxembourg | Nicorette Fruit & Mint Spray Buccal |
| Norway | Nicorette |
| Poland | Nicorette Cool Berry |
| Portugal | Nicorette Bucomist sabor fruta menta |
| Romania | Nicorette Berrymint |
| Slovakia | Nicorette Spray s príchuťou lesného ovocia |
| Slovenia | Nicorette z okusom gozdnih sadežev |
| Spain | Nicorette Bucomist sabor fruta menta |
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterJohnson & Johnson Consumer NV/SA Johnson & Johnson GmbH McNeil AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Nicorette Cool Berri
Alternatives to Nicorette Cool Berri in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nicorette Cool Berri in Spain
Alternative to Nicorette Cool Berri in Ukraine
Online doctors for Nicorette Cool Berri
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nicorette Cool Berri – subject to medical assessment and local rules.











