

Nebivolek

Ask a doctor about a prescription for Nebivolek

How to use Nebivolek
Package Leaflet: Information for the Patient
NebivoLEK, 5 mg, tablets
Nebivolol
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Contents of the package leaflet:
- 1. What is NebivoLEK and what is it used for
- 2. Important information before taking NebivoLEK
- 3. How to take NebivoLEK
- 4. Possible side effects
- 5. How to store NebivoLEK
- 6. Contents of the pack and other information
1. What is NebivoLEK and what is it used for
NebivoLEK contains nebivolol, a medicine that acts on the cardiovascular system, belonging to the group of selective beta-adrenergic blocking agents (i.e., with selective action on the circulatory system). The medicine prevents the acceleration of heart activity, controls the strength of heart contractions, and also has a dilating effect on blood vessels, which leads to a decrease in blood pressure. The medicine is used to treat high blood pressure (hypertension). NebivoLEK is also used as a supportive treatment for mild and moderate chronic heart failure in patients aged 70 or older.
2. Important information before taking NebivoLEK
When not to take NebivoLEK
if you are allergic (hypersensitive) to nebivolol or any of the other ingredients of this medicine (listed in section 6);
if you have one or more of the following disorders:
- low blood pressure;
- severe circulatory disorders in the limbs;
- very slow heart rate (less than 60 beats per minute);
- other severe heart rhythm disorders (e.g., second- and third-degree atrioventricular block, cardiac conduction disorders);
- heart failure that has recently occurred or worsened, or if you are receiving intravenous medicines to support heart function due to cardiogenic shock caused by acute heart failure;
- asthma or wheezing (currently or in the past);
- untreated pheochromocytoma - a tumor located in the glands above the kidney (in the adrenal glands);
- liver function disorders;
- metabolic disorders (metabolic acidosis), such as diabetic ketoacidosis.
Warnings and precautions
Before starting treatment with NebivoLEK, discuss with your doctor or pharmacist if you have or develop one of the following disorders:
abnormally slow heart rate;
chest pain caused by spontaneous contraction of the coronary arteries (so-called Prinzmetal's angina);
untreated chronic heart failure;
first-degree atrioventricular block (a type of mild conduction disorder in the heart that affects the heart rhythm);
poor circulation in the limbs, such as Raynaud's disease or intermittent claudication;
long-term respiratory disorders;
diabetes: this medicine does not affect blood glucose levels, but may mask warning signs of low blood glucose (such as palpitations, rapid heart rate) and may increase the risk of severe hypoglycemia when used with certain types of antidiabetic medicines called sulfonylureas (such as gliclazide, glibenclamide, glipizide, glimepiride, or tolbutamide);
hyperthyroidism: the medicine may mask abnormally rapid heart rate caused by this disease;
allergy: this medicine may exacerbate the reaction to pollen or other substances to which you are allergic;
psoriasis (a skin disease - flaky, pink patches on the skin) currently or in the past;
At the beginning of the treatment of chronic heart failure, you will be systematically monitored by an experienced doctor (see section 3).
Treatment should not be stopped abruptly without clear recommendation and assessment by the doctor (see section 3).
Children and adolescents
Do notrecommend the use of NebivoLEK in children and adolescents due to the lack of data on the use of nebivolol in this patient group.
NebivoLEK and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, includingthose that are available without a prescription. Some medicines cannot be taken at the same time, while others require certain changes (e.g., dosage).
Always tell your doctor about taking, in addition to NebivoLEK, any of the following medicines:
controlling blood pressure or used in heart diseases (such as amiodarone, amlodipine, cybenzoline, clonidine, digoxin, diltiazem, disopyramide, felodipine, flecainide, guanfacine, hydroquinidine, lacidipine, lidocaine, methyldopa, mexiletine, moxonidine, nicardipine, nifedipine, nimodipine, nitrendipine, propafenone, quinidine, rilmenidine, verapamil);
sympathomimetic medicines (stimulating the sympathetic nervous system and affecting heart function and circulation);
sedatives and antipsychotics (used to treat mental disorders), such as barbiturates (also used to treat epilepsy), phenothiazines (also used for nausea and vomiting), and thioridazine;
diabetes medicines, such as insulin or oral antidiabetic medicines;
antidepressants, such as amitriptyline, paroxetine, fluoxetine;
anesthetics used during surgery;
used for asthma, nasal congestion, or certain eye diseases, such as glaucoma (increased pressure in the eyeball) or dilation of the pupils;
amifostine used in cancer treatment;
baclofen used in the treatment of epilepsy.
All these medicines, like nebivolol, can affect blood pressure and/or heart function.
reducing stomach acid or used to treat stomach ulcers (antacids), i.e., cimetidine. NebivoLEK should be taken during meals, and the antacid between meals.
NebivoLEK with food and drink
NebivoLEK can be taken with or without food, but the tablet is best swallowed with water.
Pregnancy and breastfeeding
NebivoLEK should not be taken during pregnancy, unless absolutely necessary.
It is not recommended to take the medicine during breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before taking this medicine.
Driving and using machines
The medicine may cause dizziness or fatigue. If such symptoms occur, do notdrive vehicles or operate machines.
NebivoLEK contains lactose and sodium
This medicine contains lactose. If you have previously been diagnosed with intolerance to some sugars, you should contact your doctorbefore taking the medicine.
This medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means the medicine is considered "sodium-free".
3. How to take NebivoLEK
Always take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor.
NebivoLEK can be taken before, during, or after meals, or independently of meals. The tablet is best swallowed with water.
Treatment of high blood pressure (hypertension)
The usual dose is 1 tablet per day. The tablet is best taken daily at the same time.
Elderly patients and patients with kidney function disorders usually start treatment with a dose of ½ (half) tablet per day.
The therapeutic effect on blood pressure becomes noticeable after 1 to 2 weeks of treatment. Sometimes the best treatment result is achieved after 4 weeks.
Treatment of chronic heart failure
Treatment will be started and closely monitored by an experienced doctor.
The doctor will start treatment with a dose of ¼ (quarter) tablet per day. This dose may be increased after 1 to 2 weeks to ½ (half) tablet per day, then to 1 tablet per day, and finally to 2 tablets per day, until the appropriate dose for the patient is reached. At each stage, the doctor will prescribe the appropriate dose of the medicine, and you should strictly follow their recommendations.
The maximum recommended dose is 2 tablets (10 mg) per day.
At the beginning of treatment and during each dose increase, you must remain under close observation by an experienced doctor for 2 hours.
If necessary, the doctor may reduce the dose of the medicine.
Do not stop treatment abruptly, as this may worsen heart failure.
Patients with severe kidney function disorders should not take this medicine.
The medicine should be taken once a day, preferably at the same time every day.
How to divide the tablet
If your doctor has recommended taking ¼ or ½ tablet (two quarters), follow these steps:
- 1. Place the tablet on a smooth, hard surface so that the cloverleaf pattern is facing up, and the dividing lines on the tablet are aligned with the hours on a clock, i.e., 12, 3, 6, and 9 o'clock (Figure 1).

6
Figure 1 – How to place the tablet
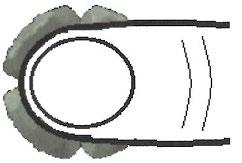
- 2. Place your thumb on the tablet, pointing in the direction of 3-9 o'clock (Figure 2)
6
Figure 2 – Placing the thumb on the tablet
- 3. Press your finger evenly on the entire surface of the tablet until it breaks.
Your doctor may decide to treat you with a combination of NebivoLEK tablets and other medicines.
Do not take the medicine in children and adolescents.
Accidental overdose of NebivoLEK
In case of accidental overdose, immediatelyinform your doctor or pharmacist. The most common symptoms of overdose are: very slow heart rate (bradycardia), low blood pressure with possible fainting (hypotension), shortness of breath, such as in asthma (bronchospasm), and acute heart failure.
While waiting for the doctor to arrive, you can take activated charcoal (available at the pharmacy).
Missing a dose of NebivoLEK
If you forget to take a dose of NebivoLEK, but remember soon after, take your usual daily dose. However, if the delay is significant (e.g., several hours) and it is almost time for the next dose, do not take the missed dose, but take the next planned doseat the usual time. Do not take a double dose. Avoid missing multiple doses of the medicine.
Stopping treatment with NebivoLEK
Before stopping treatment with NebivoLEK, always consult your doctor, regardless of whether you are taking the medicine for high blood pressure or chronic heart failure.
Do not stop treatment abruptly, as this may temporarily worsen heart failure. If stopping treatment with nebivolol for chronic heart failure is necessary, the daily dose should be gradually reduced by half every week.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, NebivoLEK can cause side effects, although not everybody gets them.
If NebivoLEK is used to treat high blood pressure, the following side effects may occur:
Common side effects (may occur in less than 1 in 10 people):
headache
dizziness
fatigue
itching or tingling sensation
diarrhea
constipation
nausea
shortness of breath
swelling of hands or feet
Uncommon side effects (may occur in less than 1 in 100 people):
slow heart rate or other heart disorders
low blood pressure
cramping leg pain when walking
vision disorders
impotence
depression
digestive disorders (indigestion), gas in the stomach or intestines, vomiting
skin rash, itching
shortness of breath (such as in asthma), caused by sudden contraction of the airway muscles (bronchospasm)
nightmares
Rare side effects (may occur in less than 1 in 10,000 people):
fainting
worsening of psoriasis (a skin disease with flaky, pink patches)
Side effects with unknown frequency (frequency cannot be estimated from available data):
hypersensitivity
angioedema (swelling of the face, lips, mouth, tongue, or throat)
urticaria (itchy rash)
The following side effects have been observed with similar medicines:
hallucinations
psychotic episodes
confusion
cold extremities, cyanosis (blue or purple discoloration of the skin)
Raynaud's phenomenon (discoloration of the skin on the fingers and toes, occasionally also on other parts of the body)
dry eyes
formation of new connective tissue in the eyes and diaphragm (ocular-mucocutaneous toxicity typical of practolol)
In a clinical study on chronic heart failure, the following side effects were observed:
Very common (may occur in more than 1 in 10 people):
slow heart rate
dizziness
Common (may occur in less than 1 in 10 people):
worsening of heart failure
low blood pressure (e.g., feeling faint when standing up quickly)
intolerance to the medicine
a type of mild conduction disorder in the heart that affects the heart rhythm (first-degree atrioventricular block)
swelling of the legs (e.g., swelling around the ankles)
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301/fax: +48 22 49 21 309/website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store NebivoLEK
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and blister after "EXP". The expiry date refers to the last day of the month.
There are no special precautions for storage.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What NebivoLEK contains
The active substance of the medicine is nebivolol. Each tablet contains 5 mg of nebivolol (as nebivolol hydrochloride).
The other ingredients are: croscarmellose sodium, lactose monohydrate, cornstarch, microcrystalline cellulose, hypromellose 5 cps, colloidal anhydrous silica, magnesium stearate.
What NebivoLEK looks like and contents of the pack
White or almost white tablets, divisible into four parts, in the shape of a cloverleaf on one side, and convex on the other side, with dividing lines marked on both sides (diameter 9 mm).
The tablets are packaged in PVC/Aluminum blisters and placed in a cardboard box.
Pack sizes: 28 or 56 tablets.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sandoz GmbH
Biochemiestrasse 10
A-6250 Kundl, Austria
Manufacturer
Lek Pharmaceuticals d.d.
Verovškova 57
1526 Ljubljana, Slovenia
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
39179 Barleben, Germany
Lek S.A.
ul. Domaniewska 50 C
02-672 Warsaw
Lek S.A.
ul. Podlipie 16
95-010 Stryków
To obtain more detailed information about the medicine and its names in other European Economic Area member states, please contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
tel. 22 209 70 00
Date of last revision of the leaflet:03/2025
Sandoz logo
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterLEK Pharmaceuticals d.d. LEK S.A. LEK S.A. Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NebivolekDosage form: Tablets, 5 mgActive substance: nebivololManufacturer: Genericon Pharma GmbH PharmaPath S.A.Prescription not requiredDosage form: Tablets, 5 mgActive substance: nebivololManufacturer: PharmaPath S.A.Prescription required
Alternatives to Nebivolek in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nebivolek in Ukraine
Alternative to Nebivolek in Spain
Online doctors for Nebivolek
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nebivolek – subject to medical assessment and local rules.










