

Natrium hloratum 0,9% Kabi

Ask a doctor about a prescription for Natrium hloratum 0,9% Kabi

How to use Natrium hloratum 0,9% Kabi
Leaflet attached to the packaging: information for the user
Sodium Chloride 0.9% Kabi, 9 mg/ml, solvent for the preparation of parenteral drugs
Sodium Chloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you personally. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Sodium Chloride 0.9% Kabi and what is it used for
- 2. Important information before using Sodium Chloride 0.9% Kabi
- 3. How to use Sodium Chloride 0.9% Kabi
- 4. Possible side effects
- 5. How to store Sodium Chloride 0.9% Kabi
- 6. Contents of the packaging and other information
1. What is Sodium Chloride 0.9% Kabi and what is it used for
Sodium Chloride 0.9% Kabi is used as a solvent for drugs administered intravenously, intramuscularly or subcutaneously, serving as a carrier for added drugs.
2. Important information before using Sodium Chloride 0.9% Kabi
When not to use Sodium Chloride 0.9% Kabi:
- if the patient is allergic or has an unusual reaction to sodium chloride;
- if the patient has high sodium levels in the blood (hypernatremia);
- if the patient has increased muscle tension (hypertonia);
- if the patient has heart failure (the heart is unable to pump the necessary amount of blood);
- if the patient has heart, liver or kidney function disorders and if there is water retention in the body;
- if the patient has high blood pressure (severe hypertension);
- if the patient has increased blood acidity (metabolic acidosis).
Warnings and precautions
Before starting to use Sodium Chloride 0.9% Kabi, discuss it with your doctor or nurse.
- After opening, the solution should be used immediately.
- In the case of subcutaneous administration, no additives that could change the isotonicity of the solution should be used.
- The solution should not be used if it is not clear and contains sediment.
- When adding a drug to the ampoule, ensure physical and chemical compatibility.
- Avoid adding alcohol to sodium chloride solutions.
Children
In newborns, there may be too high a concentration of sodium due to kidney immaturity.
Therefore, repeated injections of sodium chloride can be given after determining the sodium level in the blood.
Sodium chloride should be used with caution in patients with hypertension, heart failure, pulmonary edema or peripheral edema, kidney function disorders, pre-eclampsia, hyperaldosteronism, liver cirrhosis and other liver diseases, hypervolemia, urethral stricture, hypoproteinemia and other diseases and treatments (e.g. corticosteroids) related to sodium retention.
Sodium Chloride 0.9% Kabi and other medicines
Tell your doctor or pharmacist about all the medicines you are taking now or have taken recently, as well as any medicines you plan to take.
Interactions with other medicines depend on the medicine that may be added.
Sodium Chloride 0.9% Kabi is incompatible with hydrocortisone, amphotericin B, tetracyclines, cephalothin, erythromycin, lactobionate and lithium salts.
This medicine is incompatible with active substances insoluble in sodium chloride solution due to the possibility of precipitation of the active ingredient, as well as with medicines whose stability or solubility require a very acidic or very alkaline pH.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or plan to have a child, consult your doctor or pharmacist before using this medicine.
Given the properties of the medicine, it is not expected to have an effect on women during pregnancy or breastfeeding, provided that the administration is correct and controlled.
Driving and using machines
There is no evidence that this medicine could affect the ability to drive and use machines .
3. How to use Sodium Chloride 0.9% Kabi
Follow these instructions unless your doctor advises otherwise.
Sodium Chloride 0.9% Kabi should be administered intravenously, intramuscularly or subcutaneously.
There is no need to disinfect the ampoule before opening it.
There is no need to use any devices to open the ampoule.
After opening the ampoule, its outlet fits exactly to the end of the syringe (Luer end), so there is no need to use a needle.
Instructions for use
Twist off one ampoule by turning it in the opposite direction to the rest, without touching the end and neck of the ampoule (1). Shake the ampoule with one movement as shown below to remove the solution from the end of the ampoule (2). To open the ampoule, twist its end in the opposite direction to the rest of the ampoule until the break line (3). Connect the ampoule to a Luer or Luer-Lock syringe as shown in the picture (4).
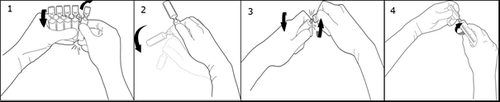
No needle is needed, take the contents of the ampoule.
The solution does not contain any preservatives or bactericidal agents, so after opening, unused ampoules should be discarded immediately.
The administered volume depends on the concentration of the drug to be dissolved.
Your doctor will inform you about the duration of treatment with these medicines.
If you feel that the effect of Sodium Chloride 0.9% Kabi is too strong or too weak, tell your doctor.
Using more than the recommended dose of Sodium Chloride 0.9% Kabi
If you have taken too much of Sodium Chloride 0.9% Kabi, tell your doctor immediately.
Due to the properties of this medicine, if it is used as indicated and administered correctly and under control, there is no risk of poisoning.
However, an excess of sodium chloride can cause dehydration of internal organs, nausea, vomiting, headache, abdominal cramps, thirst, decreased secretion of saliva, urine and sweat, fever, low blood pressure, tachycardia, kidney failure, pulmonary edema, acidosis, respiratory failure, headache, dizziness, irritability, muscle cramps, stiffness, coma and death.
If symptoms of poisoning occur, discontinue the medicine and start symptomatic treatment.
In children, coma and convulsions may persist due to dehydration of cells. Respiratory disorders with rapid breathing and redness of the nose may also occur .
In case of overdose or accidental ingestion, go to a medical facility or call a poison control center immediately.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If the medicine is administered correctly, no side effects should occur.
Inappropriate or excessive administration of the sodium chloride solution may cause overhydration, hypernatremia, hyperchloremia and related symptoms, such as metabolic acidosis caused by decreased bicarbonate levels and the occurrence of edema.
An excess of sodium chloride may cause nausea, vomiting and headache.
Side effects may also be related to the added medicines.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Sodium Chloride 0.9% Kabi
Keep the medicine out of the sight and reach of children.
There are no special precautions for storing the medicine.
Do not use this medicine after the expiry date stated on the packaging after “EXP”.
The expiry date refers to the last day of the specified month.
Shelf life after first opening: use immediately.
6. Contents of the packaging and other information
What Sodium Chloride 0.9% Kabi contains
- The active substance of the medicine is sodium chloride. 100 ml of the solution contains 0.9 g of sodium chloride.
- The other ingredients are: water for injections, 25% hydrochloric acid and sodium hydroxide.
100 ml contains:
Electrolytes
mmol/l
mEq/l
Sodium chloride
0.9 g
Na
154
154
Cl
154
154
Water for injections q.s.
up to 100 ml
The osmolality of the solution is 308 mOsmol/l.
What Sodium Chloride 0.9% Kabi looks like and what the packaging contains
Sodium Chloride 0.9% Kabi, solvent for the preparation of parenteral drugs, is a clear and colorless solution, free or almost free from solid particles.
Sodium Chloride 0.9% Kabi is available in the following packaging sizes:
20 ampoules of 5 ml
50 ampoules of 5 ml
20 ampoules of 10 ml
50 ampoules of 10 ml
20 ampoules of 20 ml.
Not all packaging sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
Manufacturer
FRESENIUS KABI ESPAÑA S.A.
Marina 16–18, planta 17 Torre Mapfre
08005 Barcelona
Spain
To obtain more detailed information, contact the marketing authorization holder:
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
tel.: +48 22 345 67 89
This medicinal product is authorized in the Member States of the European
Economic Area under the following names:
Belgium
Sodium Chloride 0.9% Fresenius Kabi solvent for parenteral use
Czech Republic
Sodium Chloride Kabi 0.9%
Estonia
Sodium Chloride Kabi 0.9%, solvent for parenteral use
Greece
Sodium Chloride 0.9%/Fresenius
Spain
Sodium Chloride Meinsol 9 mg/ml solvent for parenteral use
Ireland
Sodium Chloride 0.9% w/v solvent for parenteral use
Lithuania
Sodium Chloride Kabi 0.9% solvent for parenteral use
Latvia
Sodium Chloride Kabi 0.9% solvent for parenteral use
Poland
Sodium Chloride 0.9% Kabi
Romania
Physiological serum 9 mg/ml Kabi solvent for parenteral use
Slovakia
Sodium Chloride Kabi 0.9% solvent for parenteral use
Slovenia
Sodium Chloride Fresenius Kabi 9 mg/ml
Hungary
Sodium Chloride Kabi 9 mg/ml solvent for parenteral preparations
Date of last revision of the leaflet:05.05.2020
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFresenius Kabi Espana S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Natrium hloratum 0,9% KabiDosage form: Solution, 95 mg/mlActive substance: electrolytesPrescription requiredDosage form: Solvent, 9 mg/mlActive substance: electrolytesManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A.Prescription requiredDosage form: Solution, (1.5 mg + 9 mg)/mlActive substance: electrolytesPrescription required
Alternatives to Natrium hloratum 0,9% Kabi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Natrium hloratum 0,9% Kabi in Ukraine
Alternative to Natrium hloratum 0,9% Kabi in Spain
Online doctors for Natrium hloratum 0,9% Kabi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Natrium hloratum 0,9% Kabi – subject to medical assessment and local rules.














