
Moviprep

Ask a doctor about a prescription for Moviprep

How to use Moviprep
Package Leaflet: Information for the User
Moviprep, Powder for Oral Solution
Macrogol 3350 + anhydrous sodium sulfate + sodium chloride + potassium chloride + ascorbic acid +
sodium ascorbate
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Moviprep and what is it used for
- 2. Important information before taking Moviprep
- 3. How to take Moviprep
- 4. Possible side effects
- 5. How to store Moviprep
- 6. Contents of the pack and other information
1. What is Moviprep and what is it used for
Moviprep is a lemon-flavored laxative medicine consisting of four sachets.
The pack contains two large sachets (‘sachet A’) and two small sachets (‘sachet B’). All four sachets are needed to complete one treatment cycle.
Moviprep is for adults and is used to cleanse the bowel before examinations. It works by stimulating bowel movements.
2. Important information before taking Moviprep
When not to take Moviprep:
- if you are allergic (hypersensitive) to the active substances or any of the other ingredients of this medicine (listed in section 6),
- if you have a blockage in your intestines,
- if you have a perforated wall of the intestine,
- if you have gastroparesis (a condition where the stomach takes too long to empty its contents),
- if you have paralytic ileus (a condition where there is a lack of movement in the intestines, often after abdominal surgery),
- if you have phenylketonuria (a rare, inherited disorder that affects the body's ability to break down an amino acid called phenylalanine). Moviprep contains a source of phenylalanine,
- if you have a glucose-6-phosphate dehydrogenase deficiency (a rare condition that affects the red blood cells),
- if you have toxic megacolon (a serious complication of inflammatory bowel disease).
Warnings and precautions
If your overall health is poor or if you have serious medical conditions, you should be aware of the possible side effects listed in section 4. If you are unsure, consult your doctor or pharmacist.
Before taking Moviprep, tell your doctor or pharmacist if:
- you have a tendency to regurgitate food, fluids or gastric acid, or problems with swallowing (see also Moviprep with food and drink),
- you have kidney disease,
- you have heart failure, severe kidney problems or are taking medicines to control your blood pressure,
- you have heart problems, including high blood pressure, irregular heartbeat or palpitations,
- you have thyroid problems,
- you are dehydrated,
- you have had an exacerbation of inflammatory bowel disease (Crohn's disease or ulcerative colitis),
- you have epilepsy or have had seizures in the past. Moviprep should not be given to patients with impaired consciousness without medical supervision. If you experience sudden abdominal pain or rectal bleeding during or after taking Moviprep, seek medical attention immediately. If you vomit (with blood) and then experience sudden chest, neck or abdominal pain, difficulty swallowing or difficulty breathing after taking Moviprep, stop taking the medicine and seek medical attention immediately.
Children and adolescents
Children and adolescents under 18 years should not take Moviprep.
Moviprep with other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and any you plan to take.
If you are taking other oral medicines (e.g. oral contraceptives), do not take them within 1 hour of taking Moviprep, as they may not work as well.
Moviprep with food and drink
Do not eat solid foods from the start of Moviprep treatment until after the examination.
If you need to thicken fluids to swallow them safely, Moviprep may counteract the thickening effect.
While taking Moviprep, drink plenty of fluids. The fluid in the Moviprep solution does not replace your regular fluids.
Pregnancy, breastfeeding and fertility
There is no experience with the use of Moviprep in pregnancy or breastfeeding. The medicine should only be used if your doctor considers it necessary. If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice.
Driving and using machines
Moviprep does not affect the ability to drive or use machines.
Moviprep contains sodium, potassium and a source of phenylalanine
This medicine contains 8.4 g of sodium (the main component of common salt) per treatment cycle (two liters of prepared Moviprep solution). This is 420% of the recommended maximum daily intake of sodium for an adult. This should be taken into account for patients on a controlled sodium diet. Only part of the sodium is absorbed (up to 2.6 g per treatment cycle).
This medicine contains 1.1 g of potassium per treatment cycle (two liters of prepared Moviprep solution). This should be taken into account for patients with kidney problems or those on a controlled potassium diet.
This medicine contains a source of phenylalanine. It may be harmful to patients with phenylketonuria.
3. How to take Moviprep
Always take this medicine exactly as your doctor has told you. If you are unsure, ask your doctor or pharmacist.
The recommended dose is two liters of solution, prepared as follows:
The pack contains two transparent bags, each containing one pair of sachets: sachet A and sachet B. Each pair of sachets (A and B) should be dissolved in water to make one liter of solution. This pack is therefore sufficient to make two liters of Moviprep solution.
Before taking Moviprep, read the instructions below carefully. You need to know:
- When to take Moviprep
- How to prepare Moviprep
- How to take Moviprep
- What to expect after taking the medicine
When to take Moviprep
Always take this medicine exactly as described in the package leaflet or as your doctor has told you. If you are unsure, ask your doctor. The treatment with Moviprep must be completed before the examination starts.
The medicine can be taken either as divided doses or as a single dose, as described below:
For procedures under general anesthesia:
- 1. Divided doses: one liter of Moviprep solution the evening before the examination and one liter of Moviprep solution early in the morning of the examination day. Ensure that you stop taking Moviprep and other clear fluids at least two hours before the start of the examination.
Take the Moviprep solution and other clear fluids until at least two hours before the start of the examination.
- 2. Single dose: two liters of Moviprep solution the evening before the examination or two liters of Moviprep solution in the morning of the examination day. Ensure that you stop taking Moviprep and other clear fluids at least two hours before the start of the examination.
For procedures without general anesthesia:
- 1. Divided doses: one liter of Moviprep solution the evening before the examination and one liter of Moviprep solution early in the morning of the examination day. Ensure that you stop taking Moviprep and other clear fluids at least one hour before the start of the examination.
- 2. Single dose: two liters of Moviprep solution the evening before the examination or two liters of Moviprep solution in the morning of the examination day. Ensure that you stop taking Moviprep at least two hours before the start of the examination and other clear fluids at least one hour before the start of the examination.
Important: Do not eat solid foods from the start of Moviprep treatment until after the examination.
How to prepare Moviprep
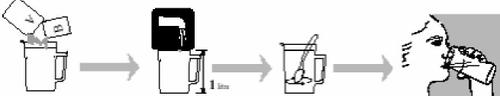
- Open one transparent bag and remove sachets A and B.
- Add the contents of BOTH sachets A and B to a measuring jug with a capacity of at least 1 liter.
- Add water to the 1-liter mark on the jug and stir until the powder is fully dissolved. The Moviprep solution should be clear or slightly cloudy. This may take up to 5 minutes.
How to take Moviprep
Drink the first liter of Moviprep solution over 1 to 2 hours. Try to drink a full glass every 10-15 minutes.
When you are ready, prepare and drink the second liter of Moviprep solution made with the contents of sachets A and B from the remaining bag.
While taking Moviprep, it is recommended to drink an additional liter of clearfluids to prevent thirst and dehydration. Suitable fluids are: water, clear soups, fruit juices (without pulp), soft drinks, tea or coffee (without milk). These fluids should be taken at least two hours before the examination under general anesthesia and at least one hour before the examination without general anesthesia.
What to expect after taking the medicine
After you start taking the Moviprep solution, stay near a toilet. You will start to have watery stools. This is normal and shows that the Moviprep solution is working. The bowel movements and the need to go to the toilet will stop soon after you finish drinking the solution. Following the instructions will cleanse your bowel so that the examination can be performed successfully. After the last fluid intake, allow sufficient time to travel to the endoscopy unit.
If you take more Moviprep than you should
Taking more Moviprep than recommended may lead to excessive diarrhea, which can result in dehydration. Drink plenty of fluids, especially fruit juices. If you are unsure, consult your doctor or pharmacist.
If you forget to take Moviprep
If you miss a dose of Moviprep, take it as soon as you remember. If it is several hours after the scheduled time, consult your doctor or pharmacist.
If you are taking Moviprep as divided doses, it is very important to finish taking the medicine at least one hour before the examination without general anesthesia or at least two hours before the examination under general anesthesia.
If you are taking Moviprep as a single dose in the morning of the examination day, it is very important to finish taking the medicine at least two hours before the examination.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Moviprep can cause side effects, although not everybody gets them.
A common symptom after taking Moviprep is diarrhea.
Stop taking Moviprep and contact your doctor immediately if you experience any of the following symptoms:
- rash or itching,
- swelling of the face, ankles or other parts of the body,
- palpitations,
- severe fatigue,
- shortness of breath.
These are symptoms of a severe allergic reaction.
Stop taking Moviprep and contact your doctor immediately if you experience any of the following side effects:
- seizures.
If you do not have a bowel movement within 6 hours of taking Moviprep, stop taking the medicine and contact your doctor immediately.
Other side effects:
Very common side effects (affecting more than 1 in 10 people):
Abdominal pain, bloating, fatigue, general malaise, anal discomfort, nausea, and fever.
Common side effects (affecting up to 1 in 10 people):
Hunger, sleep disorders, dizziness, headache, vomiting, indigestion, thirst, and chills.
Uncommon side effects (affecting up to 1 in 100 people):
Discomfort, difficulty swallowing, and changes in liver function test results.
The following side effects have also been reported, but their frequency is not known: bloating (gas), transient increase in blood pressure, irregular heartbeat or palpitations, dehydration, gagging, esophageal rupture due to vomiting, severe low sodium levels in the blood that can lead to seizures, and changes in electrolyte levels, such as decreased bicarbonate, increased or decreased calcium, increased or decreased chloride, and decreased phosphate. There may also be a decrease in potassium and sodium levels in the blood.
These reactions usually only occur during the treatment period. If they persist, consult your doctor.
Allergic reactions can cause skin rash, itching, redness, or hives, swelling of hands, feet, or ankles, headache, palpitations, shortness of breath.
Reporting of side effects
If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist. Side effects can be reported to the national reporting system via the Medicines and Healthcare products Regulatory Agency (MHRA) Yellow Card Scheme (www.mhra.gov.uk/yellowcard).
Side effects can also be reported to the marketing authorization holder.
5. How to store Moviprep
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after ‘EXP’. The expiry date refers to the last day of that month.
The batch number is stated on the carton as ‘Lot’.
Store the Moviprep sachets at room temperature (below 25°C).
After dissolving the Moviprep sachets in water, the solution can be stored (covered) at room temperature (below 25°C) or in the refrigerator (2°C-8°C). Do not store the solution for more than 24 hours.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Moviprep contains
Sachet Acontains the following active substances:
Macrogol (also known as polyethylene glycol) 3350
100 g
Anhydrous sodium sulfate
7,500 g
Sodium chloride
2,691 g
Potassium chloride
1,015 g
Sachet Bcontains the following active substances:
Ascorbic acid
4,700 g
Sodium ascorbate
5,900 g
The electrolyte concentration after dissolving both sachets in one liter of water is:
Sodium
181.6 mmol/l (of which up to 56.2 mmol is absorbable)
Chloride
59.8 mmol/l
Sulfate
52.8 mmol/l
Potassium
14.2 mmol/l
Ascorbate
56.5 mmol/l
The other ingredients are:
Lemon flavor (containing maltodextrin, citral, lemon oil, lime oil, arabic gum, vitamin E) and aspartame (E951) and acesulfame potassium (E950) as sweeteners.
For further information, see section 2.
What Moviprep looks like and contents of the pack
The pack contains two transparent bags, each containing one pair of sachets: sachet A and sachet B. Each pair of sachets (A and B) should be dissolved in one liter of water.
Moviprep powder for oral solution in sachets is available in packs containing 1 treatment set. Not all pack sizes may be marketed.
Marketing authorization holder
Norgine BV
Antonio Vivaldistraat 150
1083HP Amsterdam
Netherlands
Tel: +31 (0)20 567 09 00
Manufacturer
Norgine BV
Antonio Vivaldistraat 150
1083HP Amsterdam
Netherlands
Recipharm Höganäs AB
Sporthallsvägen 6
Höganäs, 263 35
Sweden
Sophartex
21 rue du Pressoir
28500 Vernouillet
France
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, United Kingdom (Northern Ireland): MOVIPREP
Sweden: MOVPREP
Date of last revision of the package leaflet:January 2025
The following information is intended for healthcare professionals only:
Information intended for healthcare professionals only:
Moviprep should be used with caution in patients who are seriously weakened, in poor general condition and patients with clinical disorders such as:
- impaired gag reflex or tendency to regurgitate or aspirate food
- impaired consciousness
- severe renal impairment (creatinine clearance <30 ml min)< li>
- heart failure (NYHA III or IV)
- patients at risk of arrhythmias, e.g. patients being treated for cardiovascular diseases or with thyroid disease
- dehydration
- severe exacerbation of inflammatory bowel disease
Dehydration or electrolyte disturbances should be corrected before administration of Moviprep. Patients who are partially conscious or at risk of regurgitation or aspiration should be closely monitored while taking the medicine, especially if given via a nasogastric tube. Moviprep should not be given to unconscious patients.
- Country of registration
- Prescription requiredYes
- Manufacturer
- ImporterNorgine B.V. Recipharm Hoganas AB Sophartex
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MoviprepDosage form: Powder, 13.7 gActive substance: macrogol, combinationsPrescription not requiredDosage form: Powder, -Active substance: macrogol, combinationsManufacturer: Sigma Italia S.p.A.Prescription requiredDosage form: Tablets, 1500 mgActive substance: sodium phosphatePrescription required
Alternatives to Moviprep in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Moviprep in Hiszpania
Online doctors for Moviprep
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Moviprep – subject to medical assessment and local rules.














