
Mirtagen
Ask a doctor about a prescription for Mirtagen

How to use Mirtagen
Package Leaflet: Information for the User
Warning!
The leaflet must be kept. Information on the immediate packaging in a foreign language.
Mirtagen (Mirtazapine SmeltTab Mylan), 15 mg, orodispersible tablets
Mirtazapine
Mirtagen and Mirtazapine SmeltTab Mylan are different trade names for the same medicine.
Before taking the medicine, the patient should carefully read the contents of the leaflet, as it contains important information for the patient.
- The leaflet should be kept so that it can be read again if necessary.
- In case of any doubts, the patient should consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet:
- 1. What is Mirtagen and what is it used for
- 2. Important information before taking Mirtagen
- 3. How to take Mirtagen
- 4. Possible side effects
- 5. How to store Mirtagen
- 6. Contents of the pack and other information
1. What is Mirtagen and what is it used for
Mirtagen belongs to a group of medicines called antidepressants. Mirtagen is used in the treatment of depression in adults.
2. Important information before taking Mirtagen
When not to take Mirtagen:
- if the patient is allergic to mirtazapine or any of the other ingredients of this medicine (listed in section 6),
- if the patient is currently taking or has taken in the last two weeks monoamine oxidase inhibitors (MAOIs).
Warnings and precautions
When not to take Mirtagen or consult a doctor before starting treatment:
If the patient has ever experienced symptoms such as severe skin rash or peeling, blisters, and/or ulcers of the mucous membranes of the mouth after taking Mirtagen or other medicines. During treatment with Mirtagen, severe skin reactions such as Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms have been reported. If any of the symptoms described in section 4 regarding severe skin reactions occur, the patient should stop taking the medicine and consult a doctor immediately. If the patient has ever experienced severe skin reactions, they should not restart treatment with Mirtagen.
Children and adolescents
Mirtagen should not be used in children and adolescents under 18 years of age, as its efficacy has not been established. It should also be noted that patients under 18 years of age who take medicines of this type are at increased risk of side effects such as suicidal attempts, suicidal thoughts, and hostility (especially aggression, rebellious behavior, and anger). However, the doctor may prescribe Mirtagen to patients under 18 years of age if they consider it to be in their best interest. If the doctor has prescribed Mirtagen to a patient under 18 years of age and the patient wants to discuss this, they should consult their doctor again. If the patient under 18 years of age who is taking Mirtagen develops or worsens the above symptoms, they should inform their treating doctor. Additionally, the long-term effects of Mirtagen on safety regarding growth, maturation, and cognitive and behavioral development in this age group have not been established. Furthermore, significant weight gain has been more frequently observed in patients treated with mirtazapine in this age group compared to adults.
Suicidal thoughts and worsening of depression
Patients with depression may sometimes have thoughts of self-harm or suicidal thoughts. Such thoughts may worsen when antidepressant medicines are first taken, as these medicines take time to work, usually about 2 weeks, or sometimes longer.
The patient may be particularly at risk:
- If the patient has previously had thoughts of self-harm or suicide.
- If the patient is a young adult. Information from clinical trials has shown an increased risk of suicidal behavior in adults under 25 years of age with mental disorders treated with antidepressants.
In case of suicidal thoughts or suicidal thoughts, the patient should immediately contact their doctor or go to the hospital.
Talking to a close family member or friend can be helpful.The patient can inform these people about their depression and ask them to read this leaflet so that they can tell the patient if they notice a worsening of their condition or unusual changes in behavior.
Before starting treatment with Mirtagen, the patient should discuss the following conditions with their doctor or pharmacist if they occur or have occurred:
- seizures(epilepsy),
- liver disease, including jaundice,
- kidney disease,
- heart disease or a family history of heart disease, including certain heart rhythm disorders that can cause changes in heart rhythm, recent myocardial infarction, heart failure, or the use of certain medicines that can cause heart rhythm disorders,
- low blood pressure,
- schizophrenia,
- bipolar affective disorder(alternating periods of elevated mood and depression),
- diabetes(may require a change in insulin dose or other hypoglycemic medicines),
- eye diseases, such as increased intraocular pressure (glaucoma),
- difficulty urinating, which may be caused by an enlarged prostate.
Elderly patients
- if the patient is elderly. The patient may be more sensitive to the side effects of antidepressant medicines.
During treatment
The patient should inform their doctor:
- in case of symptoms of infection, such as high fever, sore throat, and mouth ulcers. In rare cases, these symptoms may be a sign of
blood disorders. Although rare, these symptoms usually occur within 4-6 weeks of treatment.
Mirtagen and other medicines
Do not take Mirtagen with:
- monoamine oxidase inhibitors (MAOIs)or within two weeks of stopping their use. Mirtagen should not be started until two weeks after stopping MAOIs. Examples of MAOIs include moklobemide, tranylcypromine (antidepressants), and selegiline (used in the treatment of Parkinson's disease).
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription, especially:
- other antidepressant medicines like SSRIs(e.g., citalopram), venlafaxine, L-tryptophan, or triptans(used to treat migraines), tramadol(a pain reliever), linezolid(an antibiotic), lithium salts(used to treat certain psychiatric disorders), methylene blue(used to treat high levels of methemoglobin in the blood), and products containing St. John's Wort (Hypericum perforatum)(herbal products used to treat depression). In very rare cases, patients taking only Mirtagen or in combination with these medicines may experience serotonin syndrome. Some of its symptoms include fever, sweating, increased heart rate, diarrhea, (uncontrolled) muscle contractions, shivering, increased reflexes, restlessness, mood changes, and loss of consciousness. The patient should immediately contact their doctor if they experience several of these symptoms at the same time.
- medicines used to treat anxiety and insomnia, such as benzodiazepines, e.g., diazepam, chlordiazepoxide.
- medicines used to treat schizophrenia, such as olanzapine.
- medicines used to treat allergies, such as cetirizine.
- medicines used to treat severe pain, such as morphine. Mirtagen in combination with these medicines may increase the sedation caused by these medicines.
Medicines that increase the level of mirtazapine in the blood:
- medicines used to treat infections; antibacterial medicines (such as erythromycin), antifungal medicines (such as ketoconazole), medicines used to treat HIV and AIDS (such as HIV protease inhibitors, e.g., ritonavir, nelfinavir); antidepressant medicines(such as nefazodone) and medicines used to treat stomach ulcers (such as cimetidine). In combination with Mirtagen, these medicines may increase the level of mirtazapine in the blood. The patient should inform their doctor about taking these medicines. It may be necessary to reduce the dose of Mirtagen, and after stopping treatment with these medicines, the dose of Mirtagen may need to be increased.
Medicines that decrease the level of mirtazapine in the blood:
- carbamazepine and phenytoin, medicines used to treat epilepsy; rifampicin, a medicine used to treat tuberculosis. In combination with Mirtagen, these medicines may decrease the level of Mirtagen in the blood. The patient should inform their doctor about taking these medicines. It may be necessary to increase the dose of Mirtagen, and after stopping treatment with these medicines, the dose of Mirtagen may need to be decreased.
- warfarin, a medicine that prevents blood clots. Mirtagen may increase the effect of warfarin. The patient should inform their doctor about taking these medicines. In the case of combination treatment with Mirtagen, it is recommended to monitor blood tests.
Mirtagen and alcohol
Drinking alcohol while taking this medicine may cause drowsiness.
It is best to avoid drinking alcohol while taking Mirtagen.
Pregnancy and breastfeeding
Limited experience with the use of Mirtagen in pregnant women does not indicate an increased risk. However, caution should be exercised when using it during pregnancy.
If the patient becomes pregnant or plans to become pregnant while taking Mirtagen, they should ask their doctor if they can continue taking the medicine. If Mirtagen is taken during pregnancy or shortly before delivery, it is recommended to monitor the newborn for possible side effects.
The patient should ensure that their midwife and/or doctor know that they are taking Mirtagen.
The use of similar medicines (SSRIs) during pregnancy may increase the risk of a serious condition in children called persistent pulmonary hypertension of the newborn (PPHN), which causes rapid breathing and cyanosis. Symptoms usually start within 24 hours of birth. If the child experiences these symptoms, the patient should immediately contact their midwife or doctor.
The patient should ask their doctor if they can breastfeed while taking Mirtagen.
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking this medicine.
Driving and using machines
Mirtagen may reduce alertness and ability to concentrate. Before driving or operating machinery, the patient should make sure that their ability to concentrate and alertness have not been impaired.
Mirtagen contains aspartame (E 951) and mannitol (E 421)
This medicine contains 3 mg of aspartame in each orodispersible tablet. Aspartame is a source of phenylalanine. It may be harmful to patients with phenylketonuria. This is a rare genetic disorder in which phenylalanine accumulates in the body due to its improper excretion.
This medicine also contains mannitol. It may cause a mild laxative effect.
3. How to take Mirtagen
This medicine should always be taken as directed by the doctor or pharmacist. In case of doubts, the patient should consult their doctor or pharmacist.
Mirtagen is available in the following strengths: 15 mg, 30 mg, 45 mg.
Dosage
The recommended initial dose is 15 or 30 mg per day.After a few days of treatment, the doctor may recommend increasing the dose to the most suitable for the patient (from 15 to 45 mg per day).
The recommended dose is usually the same for all age groups. However, in elderly patients or those with kidney and liver disease, the doctor may recommend a different dose.
When to take Mirtagen
Mirtagen should be taken every day at the same time.
Mirtagen is best taken in a single dose, before bedtime. However, the doctor may recommend taking the medicine in two divided doses – one dose in the morning and the second dose in the evening before bedtime. The larger dose should be taken before bedtime.
Information on taking orodispersible tablets:
Tablets should be taken orally.
1. Do not crush the orodispersible tablet
To avoid crushing the orodispersible tablet, do not press on the blister (Figure A).
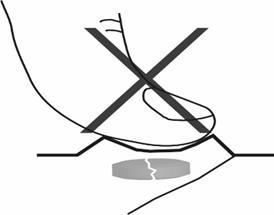
Figure A.
2. Separate the blister from the tablet
Each blister contains tablets separated by a perforated line. The patient should separate one blister along the perforated line (Figure 1).
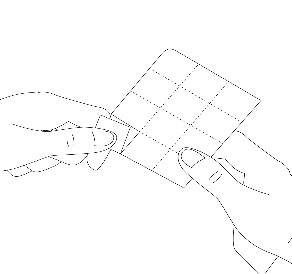
Figure 1.
3. Remove the foil
Carefully remove the covering foil, starting from the corner (Figure 2).
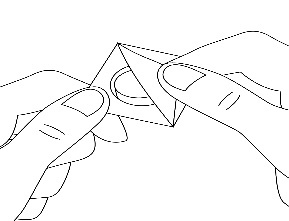
Figure 2.
4. Remove the orodispersible tablet
Remove the orodispersible tablet with dry hands and place it on the tongue. (Figure 3).
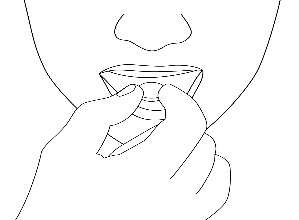
Figure 3.
The tablet dissolves quickly and can be swallowed without water.
When can improvement in condition be expected
Mirtagen usually starts working after 1 to 2 weeks, and after 2 to 4 weeks, the patient's condition will start to improve.
It is essential to discuss the effects of Mirtagen with the doctor during the first weeks of treatment.
After 2 to 4 weeks from the start of Mirtagen treatment, the patient should discuss their progress with their doctor.
If there is no adequate clinical response, the doctor may increase the dose. After another 2-4 weeks, the patient should discuss their progress with their doctor again. Treatment should be continued until the symptoms have completely disappeared, which usually takes 4 to 6 months.
Use in children and adolescents under 18 years of age
Mirtagen should not be used in children and adolescents under 18 years of age (see section 2 "Children and adolescents").
Taking a higher dose of Mirtagen than recommended
If the patient or anyone else takes a higher dose of Mirtagen than recommended, they should immediately contact their doctor.
The most common symptoms of Mirtagen overdose (without other medicines or alcohol) are drowsiness, disorientation, changes in heart rhythm (accelerated heart rate, irregular heart rhythm), and/or fainting. These may be symptoms of life-threatening ventricular arrhythmias known as "torsades de pointes".
Missing a dose of Mirtagen
In case of forgetting to take a dose of the medicine that is taken once a day.
The patient should not take the missed dose of Mirtagen; it should be skipped.
The next dose should be taken the next day at the usual time.
The patient should not take a double dose to make up for the missed tablet.
In case of forgetting to take a dose of the medicine that is taken twice a day.
If the patient forgets to take the morning dose, they should take it together with the evening dose.
If the patient forgets to take the evening dose, they should not take it together with the morning dose; it should be skipped and the patient should continue treatment, taking the usual morning and evening doses.
If the patient forgets to take both doses, they should not try to make them up; they should be skipped. The next day, the patient should continue treatment, taking the usual morning and evening doses.
Stopping treatment with Mirtagen
The patient should only stop taking Mirtagen after consulting their doctor.
The patient should not stop taking the medicine too early, as this may cause the illness to recur. If the patient's condition improves, they should discuss this with their doctor. The doctor will inform them when they can stop treatment. Suddenly stopping treatment with Mirtagen may cause nausea, dizziness, agitation, or anxiety, headache. These symptoms can be avoided by gradually stopping the medicine. The doctor will inform the patient how to gradually reduce the dose of Mirtagen.
In case of any further doubts about taking this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Mirtagen can cause side effects, although not everybody gets them.
If any of the following symptoms occur, the patient should stop taking Mirtagen and immediately contact their doctor or go to the emergency room:
Very rare
(may affect up to 1 in 1000 people):
- pancreatitis, causing moderate to severe abdominal pain radiating to the back.
Frequency not known(frequency cannot be estimated from the available data):
- seizures (convulsions),
- yellowing of the skin or eyes, which may indicate liver function disorders (jaundice),
- a combination of symptoms such as fever of unknown origin, sweating, increased heart rate, diarrhea, (uncontrolled) muscle contractions, shivering, increased reflexes, restlessness, mood changes, and loss of consciousness. In very rare cases, these may be symptoms of serotonin syndrome.
- thoughts of self-harm or suicide or suicide attempts,
- red skin lesions on the torso in the form of target-like spots or round spots, often with blisters in the center; peeling of the skin; ulcers of the mucous membranes of the mouth, throat, nose, eyes, and genitals. This type of severe skin rash may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis).
- widespread rash, high fever, and swollen lymph nodes (DRESS syndrome or drug hypersensitivity syndrome),
- infection symptoms, such as sudden high fever, sore throat, and mouth ulcers
(agranulocytosis). Mirtazapine may cause blood disorders (bone marrow suppression). Some people become less resistant to infections because mirtazapine can cause a temporary decrease in the number of white blood cells (granulocytopenia). In rare cases, mirtazapine may also cause a decrease in the number of red and white blood cells, as well as platelets (aplastic anemia), a decrease in the number of platelets (thrombocytopenia), or an increase in the number of white blood cells (eosinophilia).
- muscle tissue breakdown, causing muscle pain, tenderness, stiffness, and/or weakness, as well as dark urine (rhabdomyolysis),
- difficulty urinating or emptying the bladder,
- lower than normal sodium levels in the blood, which may cause a feeling of weakness and disorientation with muscle pain. This may be due to the improper secretion of antidiuretic hormone, which causes water retention in the body and dilution of the blood, reducing the amount of sodium.
Other side effects:
Very common(may affect more than 1 in 10 people):
- increased appetite or weight gain,
- drowsiness,
- headache,
- dry mouth.
Common(may affect up to 1 in 10 people):
- lethargy,
- dizziness,
- seizures or tremors,
- nausea,
- diarrhea,
- vomiting,
- constipation,
- rash or skin eruptions,
- joint pain, muscle pain,
- back pain,
- dizziness or fainting when changing position quickly, e.g., when standing up (orthostatic hypotension),
- swelling (usually of the ankles or feet) due to fluid accumulation (edema),
- fatigue,
- vivid dreams,
- disorientation,
- feeling anxious,
- sleep problems,
- memory problems, which in most cases disappeared after stopping treatment.
Uncommon(may affect up to 1 in 100 people):
- feeling excited or agitated (mania),
- unusual skin sensations, e.g., burning, tingling, or numbness (paresthesia),
- restless legs syndrome,
- fainting,
- unusual sensations in the mouth (oral hypoaesthesia),
- low blood pressure,
- nightmares,
- agitation,
- seeing, feeling, or hearing things that do not exist (hallucinations),
- urgent need to move.
Rare(may affect up to 1 in 1000 people):
- muscle twitching or contractions (clonic muscle spasms),
- aggressive behavior,
- increased liver enzyme activity in blood tests.
Frequency not known(frequency cannot be estimated from the available data):
- unusual sensations in the mouth, e.g., burning, tingling, or numbness (oral paresthesia),
- mouth swelling,
- low sodium levels in the blood (hyponatremia) visible in blood tests,
- increased creatine kinase activity in the blood, visible in blood tests,
- increased saliva production,
- sleepwalking,
- speech difficulties,
- high prolactin levels in the blood (hyperprolactinemia, including symptoms such as breast enlargement and/or milk discharge from the nipple),
- prolonged, painful erection of the penis.
Additional side effects in children and adolescents
The following side effects were commonly observed in clinical trials in children under 18 years of age: hives and increased triglyceride levels in the blood.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
phone: 22 49 21 301
fax: 22 49 21 309
website: https://smz.ezdrowie.gov.pl
Reporting side effects can help gather more information on the safety of the medicine.
5. How to store Mirtagen
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the packaging.
The expiry date refers to the last day of the stated month.
There are no special precautions for storage.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Mirtagen contains
- The active substance of Mirtagen is mirtazapine. Each orodispersible tablet contains 15 mg of mirtazapine.
- The other ingredients are: crospovidone, mannitol (E 421) (see section 2 "Mirtagen contains aspartame and mannitol"), microcrystalline cellulose, aspartame (E 951) (see section 2 "Mirtagen contains aspartame and mannitol"), strawberry-guarana flavor, peppermint flavor, colloidal anhydrous silica, and magnesium stearate.
Mirtagen is an orodispersible tablet.
Mirtagen 15 mg orodispersible tablets are round, white, marked with the letter "A" on one side and "36" on the other side.
For Mirtagen 15 mg orodispersible tablets, the following packaging is available: 30 orodispersible tablets.
For more detailed information, the patient should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in the Netherlands, the country of export:
Mylan Pharmaceuticals Ltd
Damastown Industrial Park
Mulhuddart
Dublin 15
Dublin, Ireland
Manufacturer:
Gerard Laboratories
35/36 Baldoyle Industrial Estate
Grange Road
Dublin 13
Ireland
Mylan Hungary Kft.
Mylan utca 1
H-2900 Komarom
Hungary
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Netherlands marketing authorization number: RVG 34052
Parallel import authorization number: 493/15
This medicine is authorized in the Member States of the European Economic Area under the following names:
Czech Republic
Mirtazapin Viatris
Denmark
Mirtazapin Viatris 15 mg smeltetabletter
Poland
Mirtagen
Portugal
Mirtazapina Mylan 15 mg comprimido orodispersível
Spain
Mirtazapina FLAS MYLAN 15 mg comprimidos bucodispersables EFG
Netherlands
Mirtazapine SmeltTab Mylan 15 mg orodispergeerbare tablet
United Kingdom
Mirtazapine 15 mg orodispersible tablets
(Northern Ireland)
Date of leaflet approval: 01.07.2025
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Mylan Pharmaceuticals Ltd
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MirtagenDosage form: Tablets, 15 mgActive substance: mirtazapinePrescription requiredDosage form: Tablets, 30 mgActive substance: mirtazapinePrescription requiredDosage form: Tablets, 45 mgActive substance: mirtazapinePrescription required
Alternatives to Mirtagen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Mirtagen in Espanha
Alternative to Mirtagen in Ukraine
Online doctors for Mirtagen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Mirtagen – subject to medical assessment and local rules.









