
Migrenofen
Ask a doctor about a prescription for Migrenofen

How to use Migrenofen
Leaflet accompanying the packaging: patient information
Migrenofen, 10 mg, orally disintegrating tablets
Rizatriptan
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Migrenofen and what is it used for
- 2. Important information before taking Migrenofen
- 3. How to take Migrenofen
- 4. Possible side effects
- 5. How to store Migrenofen
- 6. Contents of the packaging and other information
1. What is Migrenofen and what is it used for
Migrenofen belongs to a class of anti-migraine medicines called triptans (also known as selective serotonin receptor agonists).
Migrenofen is used to relieve headache pain during a migraine attack in adults.
Taking rizatriptan reduces the swelling of blood vessels surrounding the brain (cerebral blood vessels), which causes headache pain during a migraine attack.
2. Important information before taking Migrenofen
When not to take Migrenofen:
- if the patient is allergic to rizatriptan or any of the other ingredients of this medicine (listed in section 6),
- if the patient is taking monoamine oxidase inhibitors (MAOIs) such as moclobemide, phenelzine, tranylcypromine, or pargyline (antidepressants) or linezolid (an antibiotic), or if it has not been two weeks since the patient stopped taking MAOIs,
- if the patient has severe liver or kidney problems,
- if the patient has had a stroke (cerebrovascular incident) or a transient ischemic attack (TIA),
- if the patient has moderate or severe high blood pressure or mild high blood pressure that is not adequately controlled with medication,
- if the patient has had heart problems, including heart attack or angina pectoris, or symptoms of heart disease,
- if the patient has had peripheral vascular disease,
- if the patient is currently taking ergot alkaloid derivatives such as ergotamine or dihydroergotamine for migraine headaches or methysergide for migraine prophylaxis (see "Migrenofen and other medicines" below),
- if the patient is taking another medicine belonging to the same therapeutic group, such as sumatriptan, naratriptan, or zolmitriptan for migraine headaches (see "When not to take Migrenofen" above).
In case of doubt about any of the above situations, the patient should consult a doctor or pharmacist before taking Migrenofen.
Warnings and precautions
Before starting to take Migrenofen, the patient should talk to their doctor or pharmacist if:
- the patient has any risk factors for heart disease, such as high blood pressure, diabetes, smoking, or a family history of heart disease, or is over 40 years old (for men) or postmenopausal (for women),
- the patient has liver or kidney problems,
- the patient has certain heart rhythm disorders (bundle branch block),
- the patient has any allergies,
- the patient's headaches are accompanied by dizziness, walking difficulties, lack of coordination, or weakness of the limbs (arms and legs),
- the patient is taking herbal preparations containing St. John's Wort,
- the patient has had an allergic reaction with swelling of the face, lips, tongue, and/or throat, which may cause breathing and/or swallowing difficulties (angioedema),
- the patient is taking antidepressants called selective serotonin reuptake inhibitors (SSRIs) such as sertraline, escitalopram, and fluoxetine, or serotonin and norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine and duloxetine,
- the patient has had short-term symptoms such as chest pain and tightness.
Taking Migrenofen too frequently (overuse) may cause chronic, daily headaches. In such cases, the patient should consult a doctor, as it may be necessary to stop taking Migrenofen.
The patient should tell their doctor or pharmacist about all the medicines they are taking or have recently taken, as well as any medicines they plan to take. This includes medicines available without a prescription, including herbal preparations and medicines usually taken for migraines. Migrenofen may affect the way some other medicines work, and other medicines may affect the way Migrenofen works.
Children and adolescents
Migrenofen should not be used in children and adolescents under 18 years of age, as the safety and efficacy of the medicine in this age group have not been established.
Use in patients over 65 years of age
Full studies to assess the safety and efficacy of Migrenofen in patients over 65 years of age have not been conducted.
Migrenofen and other medicines
The patient should not take Migrenofen:
- if they are currently taking a migraine medicine belonging to the class of 5-HT receptor agonists (triptans), such as sumatriptan, naratriptan, or zolmitriptan (see "When not to take Migrenofen" above),
- if they are taking a monoamine oxidase inhibitor (MAOI) such as moclobemide, phenelzine, tranylcypromine, linezolid, or pargyline, or if it has not been two weeks since they stopped taking an MAOI (see "When not to take Migrenofen" above),
- if they are taking ergot alkaloid derivatives such as ergotamine or dihydroergotamine for migraine headaches (see "When not to take Migrenofen" above),
- if they are taking methysergide for migraine prophylaxis (see "When not to take Migrenofen" above),
The patient should not take the above medicines at the same time as Migrenofen, as this may increase the risk of side effects.
After taking Migrenofen, the patient should wait at least 6 hours before taking ergot alkaloid derivatives such as ergotamine, dihydroergotamine, or methysergide.
After taking ergot alkaloid derivatives, the patient should wait at least 24 hours before taking Migrenofen.
The patient should ask their doctor for information about the risks associated with taking Migrenofen:
- if they are taking propranolol (see "Warnings and precautions"),
- if they are taking SSRIs such as sertraline, escitalopram, and fluoxetine, or SNRIs such as venlafaxine and duloxetine for depression.
The patient should tell their doctor or pharmacist about all the medicines they are taking or plan to take at the same time as Migrenofen.
This includes medicines available without a prescription.
Taking Migrenofen with food and drink
Migrenofen may start working later if taken after a meal. It is better to take the medicine on an empty stomach, but it can also be taken after a meal.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before taking this medicine.
Available data on the safety of rizatriptan during the first 3 months of pregnancy do not indicate an increased risk of congenital malformations. It is not known whether Migrenofen is harmful to the unborn baby if taken by a pregnant woman after the first 3 months of pregnancy.
If the patient is breastfeeding, they should stop breastfeeding for 12 hours after taking Migrenofen to avoid exposing the baby.
Driving and using machines
During treatment with Migrenofen, the patient may experience drowsiness or dizziness. If these symptoms occur, the patient should not drive, use tools, or operate machinery.
3. How to take Migrenofen
Migrenofen is used to treat migraine headache attacks. Migrenofen should be taken as soon as possible after the migraine headache starts. The medicine should not be used to prevent migraines.
This medicine should always be taken exactly as prescribed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
The recommended dose for adults (over 18 years of age) is one orally disintegrating tablet (10 mg).
Patient taking propranolol or with liver or kidney disease should take rizatriptan at a dose of 5 mg. The patient should wait at least two hours between taking propranolol and Migrenofen and should not take more than 2 doses in 24 hours.
If the migraine returns within 24 hours
In some patients, migraine symptoms may return within 24 hours. If the migraine returns, the patient can take another dose of Migrenofen. The patient should always wait at least 2 hours between doses. The patient should not take more than 2 doses of the medicine in 24 hours.
If the migraine does not improve after 2 hours
If Migrenofen does not work, the patient should not take another dose of the medicine during the same migraine attack. However, the patient may respond to Migrenofen during the next migraine headache attack.
The patient should not take more than 2 doses of Migrenofen in 24 hours (the patient should not take more than 2 tablets of 10 mg in 24 hours). The patient should always wait at least 2 hours between doses.
If the patient's migraines worsen, they should contact their doctor.
How to take Migrenofen orally disintegrating tablets.
- Migrenofen is available as orally disintegrating tablets. One tablet contains 10 mg of rizatriptan.
- The sachet containing the tablet should be opened with dry hands.
- The tablet should be placed on the tongue, where it will dissolve and be swallowed with saliva.
- The tablet does not need to be taken with liquid. The tablet can be used in situations where liquid is not available or to avoid nausea and vomiting that may occur after taking the tablet. If necessary, the patient can drink liquid after taking the tablet.
Overdose of Migrenofen
In case of overdose, the patient should immediately consult a doctor or pharmacist. The patient should bring the packaging of the medicine with them.
After overdose, symptoms such as dizziness, drowsiness, vomiting, fainting, and slow heart rate may occur.
In case of any further doubts about the use of this medicine, the patient should consult a doctor or pharmacist.
Instructions for use
Important: Do not touch the tablet with wet hands!
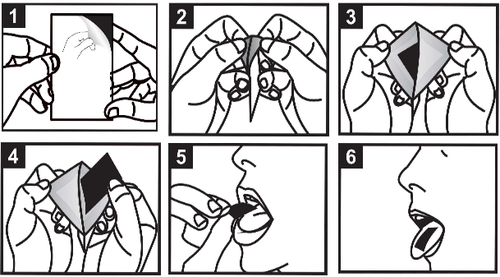
Method of use:
Step 1. Hold the sachet with the back side facing up. Find the arrow on the side, in the top corner of the sachet, and hold the sachet so that the side with the arrow is facing up. This side of the sachet is only partially glued. The two corners of the sachet are not glued, allowing the sachet to be opened.
Step 2. Hold the two unglued corners of the sachet at the point indicated by the arrow. Gently pull the two corners of the sachet in opposite directions, opening the sachet.
Step 3. Continue this movement until the two walls of the sachet are almost completely separated. The tablet will appear inside the sachet. There is no need to completely tear the sachet to access the tablet with the medicine.
Steps 4, 5, and 6. Gently remove the tablet from the sachet and place it on the tongue. The tablet will dissolve within a few seconds.
4. Possible side effects
Like all medicines, Migrenofen can cause side effects, although not everybody gets them.
The medicine may cause the following side effects.
The most common side effects reported in clinical trials with Migrenofen in adults are dizziness, drowsiness, and weakness and/or fatigue.
Common side effects (occurring in 1 to 10 out of 100 patients)
- drowsiness, tingling (paresthesia), dizziness, headache, decreased skin sensitivity to touch (hypoesthesia), impaired mental performance, insomnia, weakness, fatigue,
- rapid or irregular heartbeat (palpitations),
- short-term flushing of the face,
- unpleasant sensation (discomfort) in the throat,
- nausea, dry mouth, vomiting, diarrhea, indigestion (dyspepsia),
- feeling of heaviness in some parts of the body, neck pain, stiffness,
- abdominal pain or chest pain.
Uncommon side effects (occurring in 1 to 10 out of 1000 patients)
- taste disorders or unpleasant taste in the mouth,
- ataxia, blurred vision, tremors, fainting,
- disorientation, nervousness,
- high blood pressure (hypertension), feeling of thirst, hot flashes, flushing of the face, sweating,
- rash, itching, and papular rash (hives), swelling of the face, lips, tongue, and/or throat, which may cause breathing and/or swallowing difficulties (angioedema), breathing difficulties (dyspnea),
- feeling of pressure in some parts of the body, muscle weakness,
- changes in heart rhythm or frequency (arrhythmia), abnormalities in the electrocardiogram (a test that assesses the electrical activity of the heart), very rapid heart rate (tachycardia),
- facial pain, muscle pain.
Rare side effects (occurring in 1 to 10 out of 10,000 patients)
- wheezing,
- allergic reaction (hypersensitivity), severe life-threatening allergic reaction (anaphylaxis),
- stroke (usually occurs in patients with risk factors for heart and blood vessel disease - high blood pressure, diabetes, smoking, or a family history of heart disease),
- slow heart rate (bradycardia).
Frequency not known (frequency cannot be estimated from the available data):
- heart attack, coronary artery spasm (especially in patients with risk factors for heart and blood vessel disease such as high blood pressure, diabetes, smoking, or a family history of heart disease),
- serotonin syndrome, which may cause symptoms such as coma, unstable blood pressure, high body temperature, lack of muscle coordination, agitation, and hallucinations,
- severe skin peeling with fever or without fever (toxic epidermal necrolysis),
- seizures (convulsions),
- spasm of the blood vessels of the hands and feet, including Raynaud's phenomenon and finger or toe numbness,
- spasm of the blood vessels of the large intestine (colon), which may cause abdominal pain.
In case of symptoms of an allergic reaction, serotonin syndrome, heart attack, or stroke, the patient should immediately consult a doctor.
The patient should also immediately contact their doctor if they experience any symptoms indicating an allergic reaction (such as rash or itching) after taking Migrenofen.
If the patient experiences any side effects or if their side effects worsen, they should consult their doctor.
Reporting side effects
If the patient experiences any side effects, including those not listed in the leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: 22 49-21-301
fax: 22 49-21-309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Migrenofen
The medicine should be stored out of sight and reach of children.
There are no special precautions for storing the medicine.
Do not use this medicine after the expiry date stated on the carton/sachet after "EXP". The expiry date refers to the last day of the month stated.
Do not remove the orally disintegrating tablet from the outer aluminum sachet until the patient is ready to take the medicine. Do not use the medicine if the aluminum sachet is damaged.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Migrenofen contains
The active substance of Migrenofen is rizatriptan. Each orally disintegrating tablet contains 14.530 mg of rizatriptan benzoate (which corresponds to 10 mg of rizatriptan).
The other ingredients of Migrenofen orally disintegrating tablets are:
Hypromellose
Glycerol
Propylene glycol (E 1520)
Titanium dioxide (E 171)
Sucralose
Peppermint oil with reduced menthol content
What Migrenofen looks like and what the packaging contains
Migrenofen 10 mg is a white, rectangular, non-transparent, non-sticky orally disintegrating tablet.
Each tablet is packaged in a sachet made of LDPE/Aluminum/PET. The sachets are placed in a cardboard box.
Pack size: 2 x 1 tablet
Marketing authorization holder and importer
Marketing authorization holder
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
tel. 22 732 77 00
Importer:
Adamed Pharma S.A.
ul. Marszałka Józefa Piłsudskiego 5
95-200 Pabianice
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAdamed Pharma S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MigrenofenDosage form: Tablets, 12.5 mgActive substance: almotriptanManufacturer: Saneca Pharmaceuticals a.s.Prescription requiredDosage form: Tablets, 12.5 mgActive substance: almotriptanPrescription requiredDosage form: Tablets, 12.5 mgActive substance: almotriptanPrescription required
Alternatives to Migrenofen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Migrenofen in Ukraine
Alternative to Migrenofen in Spain
Online doctors for Migrenofen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Migrenofen – subject to medical assessment and local rules.














