
Metafen Paracetamol Max

Ask a doctor about a prescription for Metafen Paracetamol Max

How to use Metafen Paracetamol Max
Package Leaflet: Information for the Patient
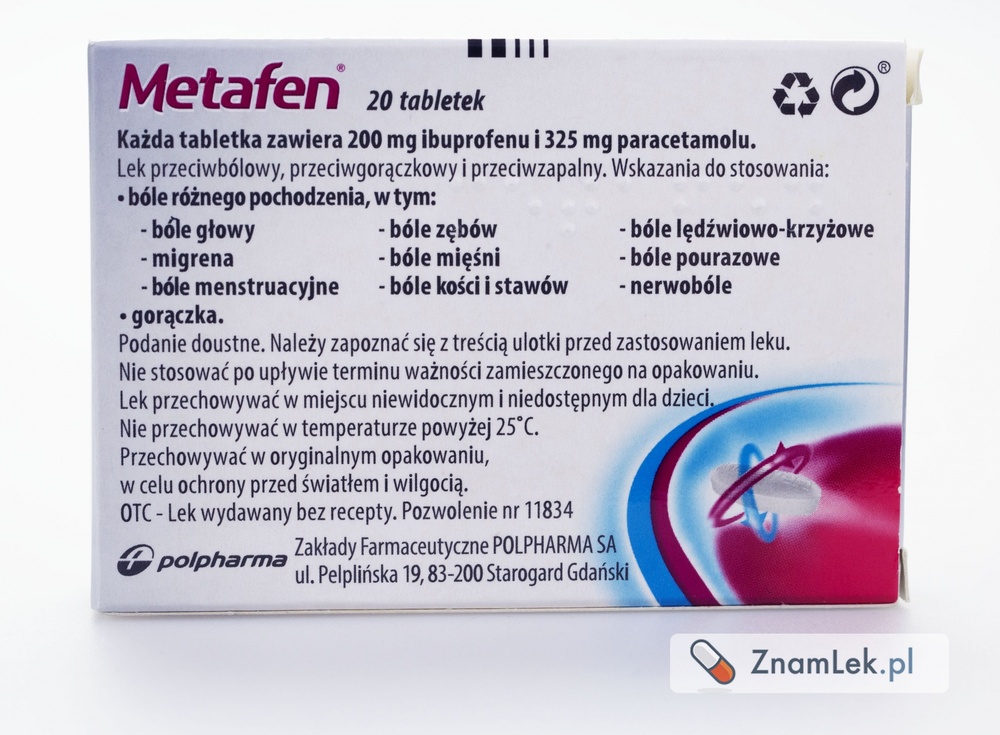
Metafen Paracetamol MAX, 1000 mg, Tablets
Paracetamol
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
This medicine should always be taken exactly as described in this package leaflet for the patient or as directed by a doctor, pharmacist, or nurse.
- Keep this package leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including those not listed in this package leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If after 3 days there is no improvement or the patient feels worse, they should contact a doctor.
Table of Contents of the Package Leaflet
- 1. What is Metafen Paracetamol Max and what is it used for
- 2. Important information before taking Metafen Paracetamol Max
- 3. How to take Metafen Paracetamol Max
- 4. Possible side effects
- 5. How to store Metafen Paracetamol Max
- 6. Contents of the pack and other information
1. What is Metafen Paracetamol Max and what is it used for
Metafen Paracetamol Max 1000 mg is a medicine with analgesic and antipyretic effects. This action is mainly due to paracetamol's ability to inhibit prostaglandin synthesis in the central nervous system. Indications for use: Pain of various origins, including:
- headaches
- migraines
- sore throats
- toothaches
- bone, joint, muscle pain
- menstrual pain In cases of colds and flu-like conditions. Fever.
If after 3 days there is no improvement or the patient feels worse, they should consult a doctor.
2. Important information before taking Metafen Paracetamol Max
When not to take Metafen Paracetamol Max
- if the patient is allergic to paracetamol or any of the other ingredients of this medicine (listed in section 6)
- in severe renal or hepatic impairment
- in cases of alcoholic liver disease.
Metafen Paracetamol Max should not be taken in children under 12 years of age.
Warnings and precautions
Read the package leaflet before taking the medicine and follow the instructions it contains. Before starting to take Metafen Paracetamol Max, discuss it with your doctor or pharmacist.
The medicine contains paracetamol.
Do not take a higher dose than recommended. Overdosing on paracetamol can lead to severe liver damage. The medicine should not be taken simultaneously with other medicines containing paracetamol, such as painkillers, antipyretics used to treat flu and cold symptoms. Before taking the medicine, consult a doctor in case of:
- liver and kidney failure,
- deficiency of certain enzymes (glucose-6-phosphate dehydrogenase and methemoglobin reductase),
- patients with malnutrition or underweight, suffering from anorexia,
- patients who regularly consume alcohol,
- (it may be necessary to completely stop taking this medicine or reduce the dose).
- severe infection (sepsis), which can increase the risk of metabolic acidosis.
During treatment with Metafen Paracetamol Max, immediately inform your doctor if the patient experiences severe diseases, including severe kidney disorders or sepsis (when bacteria and their toxins are present in the blood, leading to organ damage) or malnutrition, chronic alcoholism, or if the patient is also taking flucloxacillin (an antibiotic). In these situations, patients have been reported to develop a severe disease called metabolic acidosis (a blood and body fluid disorder), when they took paracetamol in regular doses for a longer period or when they took paracetamol with flucloxacillin. Symptoms of metabolic acidosis may include: severe breathing difficulties, including rapid deep breathing, drowsiness, feeling nauseous (nausea) and vomiting.
- deep, rapid, and difficult breathing,
- feeling nauseous and vomiting and loss of appetite
- general poor well-being.
Immediately contact a doctor if the patient experiences the above symptoms at the same time. Consult a doctor if the patient suffers from chronic headaches. During treatment, do not drink alcohol due to the increased risk of liver damage. There is a particular risk of liver damage in patients who are malnourished and regularly consume alcohol. Existing liver disease increases the risk of liver damage associated with paracetamol use. If symptoms persist, consult a doctor. Do not take a higher dose than recommended. The medicine should be stored in a place invisible and inaccessible to children.
Children under 12 years of age
Do not give Metafen Paracetamol Max to children under 12 years of age or with a body weight below 50 kg.
Metafen Paracetamol Max and other medicines
Tell your doctor, pharmacist, or nurse about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take.
Do not take other medicines containing paracetamol at the same time.
Consult a doctor when taking the following medicines:
- metoclopramide, domperidone (used against nausea and vomiting),
- cholestyramine (used to reduce high cholesterol levels in the blood)
- anticoagulant medicines such as warfarin in case of a need for long-term use of painkillers,
- sleeping pills,
- antiepileptic medicines
- antitubercular medicines,
- MAO inhibitors (medicines used in depression). Paracetamol taken with MAO inhibitors can cause a state of excitement and high temperature.
- nonsteroidal anti-inflammatory medicines (including acetylsalicylic acid)
- flucloxacillin (an antibiotic) due to the serious risk of blood and body fluid disorders (called metabolic acidosis), which must be urgently treated (see section 2).
Taking paracetamol may cause false results in some laboratory tests (e.g., blood glucose measurement).
Pregnancy, breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant, or plans to have a child, she should consult a doctor or pharmacist before taking this medicine. Metafen Paracetamol Max can be used during pregnancy if it is clinically justified. The lowest possible dose should be taken to reduce pain and (or) fever, for the shortest possible time and as infrequently as possible. The medicine should be used during pregnancy only if necessary. Paracetamol passes into breast milk in amounts that are not clinically significant. Available data do not indicate contraindications to breastfeeding during treatment with the medicine. Like other medicines, the medicine should be used during breastfeeding only if necessary.
Driving and using machines
Metafen Paracetamol Max 1000 mg taken as directed does not affect the ability to drive and use machines.
The medicine contains paracetamol
Due to the risk of overdosing, check if other medicines being taken contain paracetamol.
The medicine contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means the medicine is considered "sodium-free".
3. How to take Metafen Paracetamol Max
This medicine should always be taken exactly as described in the package leaflet for the patient or as directed by a doctor, pharmacist, or nurse. In case of doubt, consult a doctor, pharmacist, or nurse. For oral use only.
Adults and adolescents over 12 years of age (with a body weight over 50 kg)
Orally, ½ or 1 tablet, up to 4 times a day. The medicine should not be taken more frequently than every 4 hours or more than 4 tablets per day. The maximum daily dose of paracetamol is 4 g. Do not take a higher dose than recommended. Use the lowest effective dose of the medicine for the shortest possible time. The dividing line allows the tablet to be divided into two equal doses.
Children under 12 years of age (with a body weight below 50 kg)
It is not recommended to use in this patient group.
Do not take the medicine regularly for more than 3 days without consulting a doctor.
If you feel that the effect of the medicine is too strong or too weak, consult a doctor.
Taking a higher dose of Metafen Paracetamol Max than recommended
The medicine should be taken as directed. In case of taking a higher dose than recommended, immediately consult a doctor, even if the patient's condition is good and no symptoms of poisoning have been observed, due to the risk of delayed, severe liver damage, which can result in liver transplantation or death. In case of taking paracetamol in a single dose of 5 g or more and if no more than an hour has passed since ingestion, vomiting can be induced. Administer 60-100 g of activated charcoal orally, preferably mixed with water. Overdosing on paracetamol can cause symptoms such as nausea, vomiting, excessive sweating, drowsiness, and general weakness within a few to several hours. These symptoms may subside the next day, despite the fact that liver damage is beginning to develop, which will then manifest as abdominal distension, return of nausea, and jaundice.
Missing a dose of Metafen Paracetamol Max
Paracetamol is taken as needed. If a dose is missed and symptoms persist, take the next dose of the medicine. Do not take a double dose to make up for the missed dose.
Stopping treatment with Metafen Paracetamol Max
In case of further doubts about taking this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking the medicine and consult a doctor immediately if:
- an allergic reaction (hypersensitivity) occurs, such as: skin rash or itching, sometimes accompanied by difficulty breathing or swelling of the lips, tongue, throat, or face,
- a skin rash or severe skin reaction occurs, characterized by acute generalized pustular rash or blisters and erosions on the skin, mouth, eyes, and genitals, fever, and joint pain or large blisters under the skin, extensive erosions on the skin, and fever,
- breathing problems occur, especially if similar problems have occurred in the past when taking acetylsalicylic acid or other nonsteroidal anti-inflammatory medicines,
- unexplained bruising or bleeding occurs,
- liver function disorders occur.
The above side effects are very rare (occurring in less than 1 in 10,000 patients).
A serious condition called metabolic acidosis (a blood and body fluid disorder) may also occur in patients with severe disease taking paracetamol (see section 2). The frequency of this side effect is unknown (it cannot be estimated from the available data).
Reporting side effects
If any side effects occur, including those not listed in this package leaflet, inform your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Metafen Paracetamol Max
There are no special precautions for storing the medicine. The medicine should be stored in a place invisible and inaccessible to children. Do not use this medicine after the expiry date stated on the blister and carton after EXP. The expiry date refers to the last day of the month. Do not use this medicine if you notice that the blister is damaged or the appearance of the tablets has changed. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Metafen Paracetamol Max contains
- The active substance is paracetamol.
- Each tablet contains 1000 mg of paracetamol. The other excipients are: sodium carboxymethyl starch (type A), cornstarch, povidone K-30, stearic acid.
What Metafen Paracetamol Max looks like and contents of the pack
The medicine is in the form of white or almost white, elongated tablets with a dividing line on both sides, packaged in PVC/Aluminum blisters, in a cardboard box. The pack contains 10 tablets.
Marketing authorization holder and importer
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A., ul. Pelplińska 19, 83-200 Starogard Gdański, tel.: +48 22 364 61 01
Importer
Qualimetrix S.A., 579 Mesogeion Avenue, Agia Paraskevi, Athens, 15343, Greece
Date of last revision of the package leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterQualimetrix S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Metafen Paracetamol MaxDosage form: Tablets, 500 mgActive substance: paracetamolManufacturer: Farmaceutyczna Spółdzielnia Pracy "Galena"Prescription not requiredDosage form: Tablets, 300 mgActive substance: paracetamolManufacturer: Farmaceutyczna Spółdzielnia Pracy "Galena"Prescription not requiredDosage form: Tablets, 325 mgActive substance: paracetamolManufacturer: US Pharmacia Sp. z o.o.Prescription not required
Alternatives to Metafen Paracetamol Max in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Metafen Paracetamol Max in Hiszpania
Alternative to Metafen Paracetamol Max in Ukraina
Online doctors for Metafen Paracetamol Max
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Metafen Paracetamol Max – subject to medical assessment and local rules.















