

Lutinus

Ask a doctor about a prescription for Lutinus

How to use Lutinus
Package Leaflet: Information for the Patient
Warning! Keep the Leaflet! Information on the Immediate Packaging in a Foreign Language.
Lutinus, 100 mg, Vaginal Tablets
Progesteronum
Read the Leaflet Carefully Before Using the Medicinal Product, as it Contains Important Information for the Patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicinal product has been prescribed to you by a doctor for a specific reason. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet:
- 1. What is Lutinus and what is it used for
- 2. Important information before using Lutinus
- 3. How to use Lutinus
- 4. Possible side effects
- 5. How to store Lutinus
- 6. Contents of the pack and other information
1. What is Lutinus and what is it used for
Lutinus is a vaginal tablet containing the natural female sex hormone progesterone.
Lutinus is intended for women who require additional amounts of progesterone during assisted reproductive technology (ART) treatment.
Progesterone acts on the lining of the uterus, facilitating pregnancy and maintaining pregnancy in women treated for infertility.
2. Important information before using Lutinus
Lutinus should only be used in women participating in an infertility treatment program using assisted reproductive technology. Treatment begins on the day of egg retrieval. The patient is informed by the doctor about the start date of treatment.
When not to use Lutinus:
- if the patient is allergic to progesterone or any of the other ingredients of this medicinal product (listed in section 6),
- if there is non-physiological vaginal bleeding whose cause has not been explained by the doctor,
- if a miscarriage has occurred and there is a suspicion that some tissue still remains in the uterus or the pregnancy is developing outside the uterus,
- if there are or have been severe liver diseases,
- if there is a cancer or suspected cancer of the breast or genital tract,
- if there are or have been blood clots in the limbs, lungs, eyes, or other parts of the body,
- if there is porphyria (a congenital or acquired disorder of metabolism of certain enzymes).
Warnings and precautions
Before starting treatment with Lutinus, discuss it with your doctor.
Pay special attention and immediately inform your doctor if any of the following symptoms occur during treatment or even a few days after taking the last dose:
- pains in the calves or chest, sudden shortness of breath or coughing up blood, which may indicate possible blood clots in the legs, heart, or lungs,
- severe headache, vomiting, dizziness, weakness, vision or speech disorders, general weakness, or numbness of the hands or feet, which may indicate possible blood clots in the brain or eyes,
- exacerbation of depression symptoms.
Before starting treatment with Lutinus, inform your doctor if you currently have or have had any of the following diseases:
- epilepsy,
- migraine,
- asthma,
- heart or kidney disorders,
- diabetes.
Children
There is no appropriate use of Lutinus in children.
Lutinus and other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Some medicines may interact with vaginal progesterone tablets. For example, carbamazepine, rifampicin, and herbal products containing St. John's Wort may reduce efficacy, while products containing ketoconazole and vaginal antifungal creams may alter the action of progesterone.
Pregnancy and breastfeeding
Lutinus may be used in the first trimester of pregnancy in women who require additional amounts of progesterone during assisted reproductive technology treatment.
The risk of congenital anomalies (present at birth), including genital organ abnormalities in male and female children, resulting from exposure to exogenous progesterone during pregnancy, has not been fully determined.
Lutinus should not be used during breastfeeding.
Driving and using machines
Lutinus has a minor or moderate influence on the ability to drive and use machines. The medicinal product may cause drowsiness and/or dizziness, so caution is advised for drivers and machine operators.
3. How to use Lutinus
This medicinal product should always be used as directed by your doctor. In case of doubts, consult your doctor.
Usually, a dose of 100 mg is administered directly into the vagina three times a day, starting on the day of egg retrieval. If pregnancy is confirmed, Lutinus administration should be continued for 30 days.
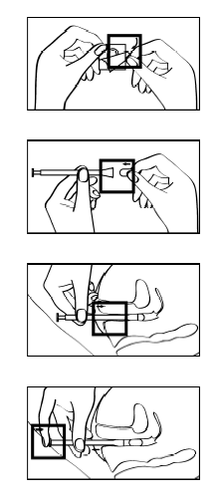
Instructions for use
Lutinus should be placed directly in the vagina using the enclosed applicator.
- 1. Remove one blister from the strip by tearing it off along the perforation.
- 2. To remove the protective foil from the bottom of the blister, start at the corner of the blister with the printed arrow.
- 3. Unpack the applicator.
- 4. Place one tablet in the designated place at the end of the applicator. The tablet should be securely placed and not fall out.
- 5. The applicator with the tablet can be inserted into the vagina in a standing, sitting, or lying position on your back with your knees bent. Gently insert the flattened end of the applicator with the tablet deep into the vagina.
- 6. Press the plunger to release the tablet from the applicator.
Withdraw the applicator, rinse it thoroughly with warm running water, dry it with a soft cloth, and keep it for reuse.
Using a higher dose of Lutinus than recommended
In case of using a higher dose of Lutinus than recommended, consult a doctor or pharmacist.
Missing a dose of Lutinus
Take the missed dose as soon as you remember, and then proceed as before. Do not take a double dose to make up for the missed dose.
Stopping treatment with Lutinus
In case of stopping or intending to stop treatment with Lutinus, consult a doctor or pharmacist. Abrupt discontinuation of progesterone may cause increased anxiety, mood swings, and increased susceptibility to seizures.
4. Possible side effects
Like all medicines, Lutinus can cause side effects, although not everybody gets them.
The most common side effects are headache, vaginal disorders, and uterine contractions.
The following, common side effectsaffect 1 to 10 in every 100 treated patients:
- headache,
- abdominal distension (abdominal swelling),
- abdominal pain,
- nausea,
- uterine contractions.
The following, uncommon side effectsaffect 1 to 10 in every 1,000 treated patients:
- dizziness,
- insomnia,
- diarrhea,
- constipation,
- hives (allergic rash),
- rash,
- vaginal disorders (e.g., discomfort in the vagina, burning sensation, discharge, dryness, and bleeding),
- vaginal fungal infection,
- breast disorders (e.g., breast pain, breast swelling, and breast tenderness),
- itching in the genital area,
- peripheral edema (edema caused by fluid accumulation).
The following side effectshave been observed after the medicinal product has been placed on the market; frequency is not known (cannot be estimated from the available data):
- fatigue
- vomiting
- allergic reactions.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicinal product.
5. How to store Lutinus
Keep the medicinal product out of the sight and reach of children.
Do not use this medicinal product after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month.
Store in the original packaging to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Lutinus contains
- The active substance of Lutinus is progesterone. One vaginal tablet contains 100 mg of progesterone.
- The other ingredients are: hydrophobic colloidal silica, lactose monohydrate, corn starch, povidone K 29/32, adipic acid, sodium bicarbonate, sodium lauryl sulfate, magnesium stearate.
What Lutinus looks like and contents of the pack
Lutinus is a vaginal tablet. It is a convex, elongated tablet, white or off-white in color, with the inscription "FPI" on one side and "100" on the other side.
Pack size: 21 or 90 vaginal tablets in Al/Al blisters and 1 polyethylene applicator in a cardboard box.
For more detailed information, contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Romania, the country of export:
Ferring GmbH
Wittland 11
D-24109 Kiel, Germany
Manufacturer:
Ferring GmbH
Wittland 11
D-24109 Kiel, Germany
Parallel importer:
InPharm Sp. z o.o.
Strumykowa 28/11, 03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
Chełmżyńska 249, 04-458 Warsaw
Marketing authorization number in Romania, the country of export:7218/2014/01
7218/2014/02
Parallel import authorization number: 384/22
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Bulgaria, Denmark, Finland, Greece, Spain, Netherlands, Ireland, Iceland, Germany, Norway, Poland, Czech Republic, Slovakia, Sweden, Hungary: Lutinus
Portugal: Luferti
Romania: Lutinus 100 mg, comprimate vaginale
Slovenia: Lutinus 100 mg vaginalne tablete
United Kingdom: Lutigest
Date of approval of the leaflet: 19.10.2022
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Ferring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LutinusDosage form: Gel, 80 mg/gActive substance: progesteronePrescription requiredDosage form: Suppositories, 400 mgActive substance: progesteroneManufacturer: Fulton Medicinali S.p.A. Gedeon Richter Plc.Prescription requiredDosage form: Suppositories, 400 mgActive substance: progesteronePrescription required
Alternatives to Lutinus in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lutinus in Spain
Alternative to Lutinus in Ukraine
Online doctors for Lutinus
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lutinus – subject to medical assessment and local rules.









