
How to use Lenalidomide Eugia
Leaflet attached to the packaging: information for the user
Lenalidomide Eugia, 5 mg, hard capsules
Lenalidomide Eugia, 10 mg, hard capsules
Lenalidomide Eugia, 15 mg, hard capsules
Lenalidomide Eugia, 25 mg, hard capsules
Lenalidomide
You should carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Lenalidomide Eugia and what is it used for
- 2. Important information before taking Lenalidomide Eugia
- 3. How to take Lenalidomide Eugia
- 4. Possible side effects
- 5. How to store Lenalidomide Eugia
- 6. Contents of the packaging and other information
1. What is Lenalidomide Eugia and what is it used for
What is Lenalidomide Eugia
Lenalidomide Eugia contains the active substance lenalidomide. This medicine belongs to a group of medicines that affect the functioning of the immune system.
What is Lenalidomide Eugia used for
Lenalidomide Eugia is used in adult patients to treat:
- multiple myeloma,
- myelodysplastic syndromes,
- mantle cell lymphoma,
- follicular lymphoma.
Multiple myeloma
Multiple myeloma is a type of cancer that attacks a certain type of white blood cell called plasma cells. These cells accumulate in the bone marrow and undergo uncontrolled divisions. This can lead to bone and kidney damage. In principle, multiple myeloma is incurable. However, it is possible to temporarily significantly alleviate or remove the signs and symptoms of the disease. We call this "remission". New diagnosis of multiple myeloma - in patients after bone marrow transplantation Lenalidomide Eugia is used without other medicines in maintenance treatment, after achieving a suitable condition after transplantation. New diagnosis of multiple myeloma - in patients who are not eligible for bone marrow transplantation treatment Lenalidomide Eugia is taken with other medicines, including:
- a chemotherapy medicine called bortezomib
- another medicine called dexamethasone
- another medicine called melphalan and
- a medicine with immunosuppressive action called prednisone. The patient starts treatment with additional medicines and then continues with Lenalidomide Eugia alone.
If the patient is 75 years old or older, or has moderate to severe kidney problems, the doctor will perform thorough tests before starting treatment. Multiple myeloma - in patients who have undergone previous treatment Lenalidomide Eugia is taken in combination with another anti-inflammatory medicine called dexamethasone. Lenalidomide Eugia may stop the progression of multiple myeloma symptoms and signs. It has also been shown to delay the recurrence of multiple myeloma after treatment.
Myelodysplastic syndromes (MDS)
Myelodysplastic syndromes (MDS) are a group of different blood and bone marrow diseases. Abnormal blood cells are present, which do not function properly. Patients may experience various subjective and objective symptoms, including a low number of red blood cells (anemia), the need for blood transfusions, and the risk of infection. Lenalidomide Eugia is used as monotherapy in the treatment of adult patients who have been diagnosed with MDS, if all of the following conditions are met:
- the patient needs regular blood transfusions due to a low number of red blood cells ("transfusion-dependent anemia")
- the patient has a cytogenetic abnormality in bone marrow cells called "isolated deletion 5q". This means that the patient's body does not produce enough healthy blood cells
- the patient has previously undergone other treatments that have proven to be ineffective or insufficient.
Taking Lenalidomide Eugia may lead to an increase in the number of healthy blood cells produced by the body, by reducing the number of abnormal cells:
- this may lead to a decrease in the number of blood transfusions required. It is possible that transfusions will no longer be needed.
Mantle cell lymphoma (MCL)
MCL is a cancer of part of the immune system (lymphoid tissue). It attacks a certain type of white blood cell called B lymphocytes or B cells. Mantle cell lymphoma is a disease characterized by uncontrolled growth of B lymphocytes, resulting in their accumulation in lymphoid tissue, bone marrow, or blood. Lenalidomide Eugia is used as monotherapy in the treatment of adult patients who have previously been treated with other medicines.
Follicular lymphoma (FL)
FL is a slow-growing malignant cancer that attacks B lymphocytes. It is a type of white blood cell that helps the body fight infections. In patients with FL, there may be an accumulation of too many B lymphocytes in the blood, bone marrow, lymph nodes, and spleen. Lenalidomide Eugia is taken with another medicine called "rituximab" in the treatment of adult patients with previously treated follicular lymphoma.
How Lenalidomide Eugia works
Lenalidomide Eugia works by affecting the functioning of the immune system and directly attacking cancer cells. The medicine works in several different ways:
- by inhibiting the growth of cancer cells
- by inhibiting the growth of blood vessels in the tumor
- by stimulating part of the immune system to attack cancer cells.
2. Important information before taking Lenalidomide Eugia
Before starting treatment with Lenalidomide Eugia, you should carefully read the leaflets of all medicinal products taken in combination with Lenalidomide Eugia.
When not to take Lenalidomide Eugia:
- If the patient is pregnant, suspects pregnancy, or plans to become pregnant, because it is expected that Lenalidomide Eugia is harmful to the unborn child(see section 2, "Pregnancy, breastfeeding, and contraceptives - information for women and men").
- If the patient may become pregnant, unless all required pregnancy prevention measures are taken (see section 2, "Pregnancy, breastfeeding, and contraceptives - information for women and men"). If the patient may become pregnant, the doctor will always make a note when prescribing the medicine that the necessary measures have been taken and will inform the patient about it.
- If the patient is allergic to lenalidomide or any of the other ingredients of this medicine (listed in section 6). In case of suspected allergy, you should consult a doctor.
If any of these points apply to the patient, they should not take Lenalidomide Eugia. In case of doubts, you should consult a doctor.
Warnings and precautions
Before starting Lenalidomide Eugia, you should discuss it with your doctor, pharmacist, or nurse if:
- the patient has a history of blood clots - this means an increased risk of developing blood clots in veins and arteries during treatment
- the patient has any symptoms of infection, such as cough or fever
- the patient currently has or has had a viral infection, especially chickenpox and shingles, hepatitis B, HIV. In case of doubts, you should consult a doctor. Treatment with Lenalidomide Eugia may cause the reactivation of viruses in patients who have been infected in the past, leading to the recurrence of the infection. The doctor will check if the patient has had hepatitis B in the past.
- the patient has kidney problems - the doctor may adjust the dose of lenalidomide
- the patient has had a heart attack, has ever had a blood clot, smokes, has high blood pressure, or high cholesterol levels
- the patient has had allergic reactions when taking thalidomide (another medicine used to treat multiple myeloma), such as rash, itching, swelling, dizziness, or difficulty breathing
- the patient has had a combination of any of the following symptoms: widespread rash, skin redness, high body temperature, flu-like symptoms, elevated liver enzyme levels, blood abnormalities (eosinophilia), swollen lymph nodes - these are symptoms of a severe skin reaction called Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) or "drug hypersensitivity syndrome" (see also section 4 "Possible side effects").
If any of the above applies to the patient, they should consult a doctor, pharmacist, or nurse before starting treatment.
Tests and examinations
Before and during treatment with Lenalidomide Eugia, the patient will undergo regular blood tests, as Lenalidomide Eugia may cause a decrease in the number of blood cells that help fight infection (white blood cells) and help blood clot (platelets). The doctor will call the patient for blood tests:
- before treatment
- every week for the first 8 weeks of treatment
- then at least once a month.
Before starting treatment with lenalidomide and during treatment, the patient may undergo an assessment for circulation and breathing problems. Patients with MDS taking Lenalidomide Eugia If the patient has myelodysplastic syndromes, there is an increased chance of developing a severe disease called acute myeloid leukemia. Additionally, it is not known how Lenalidomide Eugia affects the likelihood of developing acute myeloid leukemia. Therefore, the doctor may perform tests and check for signs that will help better predict the risk of acute myeloid leukemia during treatment with Lenalidomide Eugia. Patients with MCL taking Lenalidomide Eugia. The doctor will ask for a blood test:
- before treatment
- every week for the first 8 weeks (2 cycles) of treatment
- then every 2 weeks in cycles 3 and 4 (more information is contained in section 3 "Treatment cycle")
- then at the beginning of each cycle and
- at least once a month.
Patients with FL taking Lenalidomide Eugia. The doctor will ask for a blood test:
- before treatment
- every week for the first 3 weeks (1 cycle) of treatment
- then every 2 weeks in cycles 2 to 4 (more information is contained in section 3 "Treatment cycle")
- then at the beginning of each cycle and
- at least once a month.
The doctor may perform a test to check if the patient has a large amount of tumor tissue in the body, including bone marrow. This may lead to a situation where the tumor tissue begins to die and causes abnormal growth of various substances in the blood, which can lead to kidney failure (a condition called tumor lysis syndrome). The doctor may perform a test to check if the patient has skin changes, such as red spots or rash. The doctor may change the dose of Lenalidomide Eugia or stop treatment based on the results of the patient's blood tests and overall condition. If the disease is newly diagnosed, the doctor may also assess treatment based on the patient's age and other diseases that may have occurred in the past.
Blood donation
During treatment and for at least 7 days after stopping treatment, the patient should not donate blood.
Children and adolescents
Lenalidomide Eugia is not recommended for use in children and adolescents under 18 years of age.
Elderly and patients with kidney problems
If the patient is 75 years old or older, or has moderate to severe kidney problems, the doctor will perform thorough tests before starting treatment.
Lenalidomide Eugia and other medicines
The patient should tell their doctor or nurse about all medicines they are currently taking or have recently taken. This is necessary because Lenalidomide Eugia may affect the action of other medicines. Other medicines may also affect the action of Lenalidomide Eugia. In particular, the patient should inform their doctor or nurse if they are taking:
- certain contraceptives, such as oral contraceptives, as they may stop working
- certain medicines used for heart problems - such as digoxin
- certain medicines used to thin the blood - such as warfarin.
Pregnancy, breastfeeding, and contraceptives - information for women and men
Pregnancy
For women taking Lenalidomide Eugia
- Lenalidomide Eugia should not be taken if the patient is pregnant, as it is expected to be harmful to the unborn child.
- The patient should not become pregnant while taking Lenalidomide Eugia. Therefore, women who may become pregnant must use effective contraception (see "Contraception").
- If the patient becomes pregnant while taking Lenalidomide Eugia, they should stop treatment immediately and inform their doctor.
For men taking Lenalidomide Eugia
- If the partner of a man taking Lenalidomide Eugia becomes pregnant, she should inform her doctor immediately. The partner should consult a doctor.
- Men should also use effective contraception (see "Contraception").
Breastfeeding
Breastfeeding should not be done while taking Lenalidomide Eugia, as it is not known whether lenalidomide passes into human milk.
Contraception
Women taking Lenalidomide Eugia Before starting treatment, the patient should ask their doctor about the possibility of becoming pregnant, even if they think it is unlikely. Women who may become pregnant:
- will have pregnancy tests performed under medical supervision (before each treatment, at least every 4 weeks during treatment, and at least 4 weeks after stopping treatment), unless the patient has had their fallopian tubes cut and unblocked (tubal ligation or sterilization) AND
- must use effective contraception methods for at least 4 weeks before starting treatment, during treatment, and for 4 weeks after stopping treatment. The doctor will recommend suitable contraception methods to the patient.
Men taking Lenalidomide Eugia Lenalidomide is present in human semen. If a woman is pregnant or may become pregnant and is not using effective contraception, her partner should use a condom during treatment and for at least 7 days after stopping treatment. This also applies to men who have had a vasectomy. During treatment and for at least 7 days after stopping treatment, the patient should not donate semen.
Driving and using machines
The patient should not drive or operate machinery if they experience dizziness, fatigue, drowsiness, balance problems caused by dizziness, or blurred vision after taking Lenalidomide Eugia.
Lenalidomide Eugia contains lactose
If the patient has been diagnosed with intolerance to some sugars, they should contact their doctor before taking this medicine.
Lenalidomide Eugia contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per capsule, which means it is considered "sodium-free".
3. How to take Lenalidomide Eugia
Lenalidomide Eugia must be administered by medical personnel who have experience in treating multiple myeloma, MDS, MCL, or FL.
- In the case of using Lenalidomide Eugia in the treatment of multiple myeloma in patients who are not eligible for bone marrow transplantation or who have undergone previous treatment, the medicine is used with other medicines (see section 1 "What is Lenalidomide Eugia used for").
- In the case of using Lenalidomide Eugia in the treatment of multiple myeloma in patients after bone marrow transplantation or in the treatment of patients with MDS or MCL, the medicine is used as monotherapy.
- When Lenalidomide Eugia is used in the treatment of follicular lymphoma, it is taken with another medicine called "rituximab". Lenalidomide Eugia should always be taken as recommended by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
If the patient is taking Lenalidomide Eugia with other medicines, they should read the leaflet attached to their packaging to get information about their use and action.
Treatment cycle
Lenalidomide Eugia is taken on specific days during a period of three weeks (21 days).
- Each 21-day period is called a treatment cycle.
- Depending on the day of the cycle, the patient will take one or more medicines. However, on some days, the patient will not take any medicines.
- After completing each 21-day cycle, the patient should start a new 21-day cycle.
OR Lenalidomide Eugia is taken on specific days during a period of four weeks (28 days).
- Each 28-day period is called a treatment cycle.
- Depending on the day of the cycle, the patient will take one or more medicines. However, on some days, the patient will not take any medicines.
- After completing each 28-day cycle, the patient should start a new 28-day cycle.
Recommended dose of Lenalidomide Eugia
Before starting treatment, the doctor will inform the patient:
- how much Lenalidomide Eugia they should take
- how many other medicines they should take in combination with Lenalidomide Eugia, if other medicines are necessary
- on which days of the cycle they should take which medicines.
How and when to take Lenalidomide Eugia
- The capsule should be swallowed whole, preferably with water.
- Do not break, open, or chew the capsules. If the powder from a damaged Lenalidomide Eugia capsule comes into contact with the skin, it should be washed immediately with soap and water.
- Healthcare professionals, caregivers, and family members should wear disposable gloves when handling the blister or capsule. The gloves should then be carefully removed to avoid skin exposure, placed in a sealed plastic bag, and disposed of in accordance with local regulations. Then, the hands should be washed thoroughly with soap and water. Pregnant or potentially pregnant women should not touch the blister or capsule.
- The capsules can be taken with or without food.
- Lenalidomide Eugia should be taken at approximately the same time every day when the medicine is scheduled to be taken.
Taking this medicine
To remove the capsule from the blister:
- press the capsule only from one side and push it through the foil
- do not press the center of the capsule, as this may damage it.
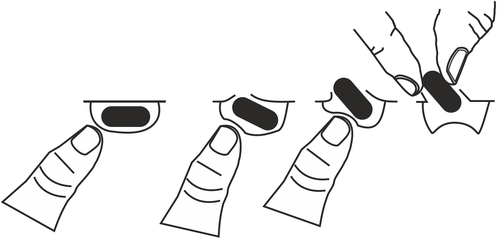
Duration of treatment with Lenalidomide Eugia
Lenalidomide Eugia is used in treatment cycles; each cycle lasts 28 days (see above "Treatment cycle"). Treatment cycles should be continued until the doctor decides to stop the medicine.
Taking a higher dose of Lenalidomide Eugia than recommended
If a higher dose of Lenalidomide Eugia than prescribed is taken, the doctor should be informed immediately.
Missing a dose of Lenalidomide Eugia
If a dose of Lenalidomide Eugia is missed at the scheduled time and
- less than 12 hours have passed since then: the capsule should be taken immediately.
- more than 12 hours have passed since then: the capsule should not be taken. The next capsule should be taken at the scheduled time the next day.
In case of any further doubts about taking this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Lenalidomide Eugia may cause side effects, although not everybody gets them.
If any of the following serious side effects occur, the patient should stop taking Lenalidomide Eugia and consult their doctor immediately - immediate treatment may be necessary:
- Hives, rash, swelling of the eyes, lips, or face, difficulty breathing, or itching, which may be symptoms of severe forms of allergic reactions called angioedema and anaphylaxis.
- A severe allergic reaction that starts as a rash in one area and spreads to the entire body, accompanied by a significant loss of skin (Stevens-Johnson syndrome and/or toxic epidermal necrolysis).
- Widespread rash, high body temperature, elevated liver enzyme levels, blood abnormalities (eosinophilia), swollen lymph nodes, and involvement of other organs (drug reaction with eosinophilia and systemic symptoms, also known as DRESS or "drug hypersensitivity syndrome") (see also section 2).
The patient should inform their doctor immediately if they experience any of the following serious side effects:
- Fever, chills, sore throat, cough, mouth ulcers, or any other symptoms of infection (including blood infections (sepsis))
- Bleeding or bruising without injury
- Chest pain or leg pain
- Shortness of breath
- Bone pain, muscle weakness, confusion, or fatigue that may be due to high calcium levels in the blood.
Lenalidomide Eugia may decrease the number of white blood cells that fight infection and the number of blood cells that help blood clot (platelets), which can lead to bleeding disorders, such as nosebleeds and bruising. Lenalidomide Eugia may also cause the formation of blood clots in the veins (thrombosis).
Other side effects
Note that in a small number of patients, Lenalidomide Eugia may cause the development of other types of cancer, and it is possible that this risk may increase with treatment with Lenalidomide Eugia. Therefore, the treating doctor should carefully assess the benefits and risks of prescribing Lenalidomide Eugia to the patient. Very commonside effects (may occur in more than 1 in 10 people):
- decrease in the number of red blood cells, which may cause anemia leading to fatigue and weakness
- rash, itching
- muscle spasms, muscle weakness, muscle pain, muscle tenderness, bone pain, joint pain, back pain, limb pain
- generalized swelling, including swelling of the hands and feet
- weakness, fatigue
- flu and flu-like symptoms, including fever, muscle pain, headache, ear pain, cough, and chills
- numbness, tingling, or burning sensation of the skin, pain in the hands or feet, dizziness, tremors
- decreased appetite, altered taste
- increased pain, tumor growth, or redness around the tumor
- weight loss
- constipation, diarrhea, nausea, vomiting, abdominal pain, heartburn
- low levels of potassium or calcium and/or sodium in the blood
- abnormal thyroid function
- leg pain (which may be a symptom of thrombosis), chest pain, or shortness of breath (which may be symptoms of blood clots in the lungs, called pulmonary embolism)
- all types of infections, including sinusitis, pneumonia, and upper respiratory tract infections
- shortness of breath
- blurred vision
- blurred vision (cataract)
- kidney problems, including abnormal kidney function or inability to maintain normal kidney function
- abnormal liver function test results
- increased liver function test values
- changes in blood proteins, leading to blood vessel inflammation (vasculitis)
- increased blood sugar levels (diabetes)
- decreased blood sugar levels
- nosebleeds
- headache
- dry skin
- depression, mood changes, sleep disturbances
- cough
- low blood pressure
- general feeling of physical discomfort, malaise
- painful inflammation of the mouth, dry mouth
- dehydration.
Commonside effects (may occur in less than 1 in 10 people):
- breakdown of red blood cells (hemolytic anemia)
- certain types of skin tumors
- bleeding from the gums, stomach, or intestines
- high blood pressure, slow, fast, or irregular heart rhythm
- increased levels of a substance produced by the breakdown of red blood cells
- increased levels of a protein that indicates inflammation in the body
- skin discoloration; skin discoloration due to bleeding under the skin, usually caused by bruising; skin swelling filled with blood, bruising
- increased uric acid levels in the blood
- skin rashes, redness, peeling, cracking, or flaking of the skin, hives
- itching, increased sweating, night sweats
- difficulty swallowing, sore throat, voice problems, or voice changes
- runny nose (rhinitis)
- passing much more or much less urine than normal or inability to control the timing of urination
- passing blood in the urine
- shortness of breath, especially when lying down (which may be a symptom of heart failure);
- erectile dysfunction
- stroke, fainting, dizziness (inner ear disorders that cause a feeling of spinning), loss of consciousness
- chest pain radiating to the arms, neck, jaw, back, or abdomen, feeling of sweating and shortness of breath, nausea, or vomiting, which may be symptoms of a heart attack (myocardial infarction)
- muscle weakness, lack of energy
- neck pain, chest pain
- chills
- joint swelling
- slowing or blocking of bile flow from the liver
- low levels of phosphate or magnesium in the blood
- speech difficulties
- liver damage
- balance problems, difficulty walking
- hearing loss, tinnitus (ringing in the ears)
- nerve pain, unpleasant, abnormal sensations, especially in response to touch
- excessive iron in the body
- thirst
- confusion
- toothache
- fall that may lead to injury.
Uncommonside effects (may occur in less than 1 in 100 people):
- bleeding inside the skull
- circulation problems
- vision loss
- loss of sex drive (libido)
- passing large amounts of urine, with accompanying bone pain and weakness, which may be symptoms of kidney disease (Fanconi syndrome)
- yellowing of the skin, mucous membranes, or eyes (jaundice), pale stools, dark urine, itching, rash, abdominal pain, or swelling - these may be symptoms of liver damage (liver failure)
- abdominal pain, bloating, or diarrhea, which may be symptoms of colon inflammation (colitis or proctitis)
- kidney cell damage (tubular necrosis)
- skin color changes, sensitivity to sunlight
- tumor lysis syndrome - metabolic complications that can occur during cancer treatment, as well as sometimes without treatment. These complications are caused by the breakdown products of dying cancer cells and can include changes in blood chemistry; high levels of potassium, phosphate, uric acid, and low levels of calcium, leading to kidney function disorders, heart rhythm disturbances, seizures, and sometimes death.
- high blood pressure in the blood vessels leading to the lungs (pulmonary hypertension).
Rareside effects (frequency cannot be estimated from available data):
- sudden or gradual, but worsening, pain in the upper abdomen and/or back, lasting for several days, most often with accompanying nausea, vomiting, fever, and rapid heart rate - these symptoms may occur in connection with pancreatitis
- wheezing, shortness of breath, or dry cough, which may be caused by lung inflammation
- rare cases of muscle breakdown (pain, weakness, or swelling of the muscles) that can lead to kidney problems (rhabdomyolysis), some of which occurred when lenalidomide was taken with a statin (a type of cholesterol-lowering medicine)
- a skin disease caused by inflammation of small blood vessels, accompanied by joint pain and fever (leukocytoclastic vasculitis)
- breakdown of the stomach or intestine wall, which can lead to a very severe infection. The patient should tell their doctor if they experience severe stomach pain, fever, nausea, vomiting, blood in the stool, or changes in bowel function
- viral infections, including chickenpox and shingles, and reactivation of hepatitis B virus (which can cause yellowing of the skin and eyes, dark urine, abdominal pain on the right side, fever, nausea, and vomiting)
- rejection of a transplanted organ (e.g., kidney, heart).
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl By reporting side effects, more information can be collected on the safety of the medicine. Side effects can also be reported to the marketing authorization holder.
5. How to store Lenalidomide Eugia
The medicine should be stored in a place that is out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton and blister after: EXP. The expiry date refers to the last day of the month. Store in a temperature below 30°C. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Lenalidomide Eugia contains
- The active substance of the medicine is lenalidomide. Each capsule contains 5 mg of lenalidomide. Each capsule contains 10 mg of lenalidomide. Each capsule contains 15 mg of lenalidomide. Each capsule contains 25 mg of lenalidomide.
- Other ingredients are: Capsule contents: lactose, microcrystalline cellulose (Type-102), croscarmellose sodium, magnesium stearate Capsule shell: titanium dioxide (E 171), yellow iron oxide (E 172) (only for 10 mg dose), indigo carmine (E 132) (only for 10 mg dose), red iron oxide (E 172) (only for 10 mg and 15 mg doses), white gelatin Ink: shellac, black iron oxide, potassium hydroxide
What Lenalidomide Eugia looks like and contents of the pack
Hard capsule. Lenalidomide Eugia, 5 mg, hard capsules: [Size approximately 17.8 mm] Hard gelatin capsules, size "2", consisting of a white opaque body and a white opaque cap with the imprint "L5" in black ink, filled with a powder that is white to pale yellow in color. Lenalidomide Eugia, 10 mg, hard capsules: [Size approximately 21.4 mm] Hard gelatin capsules, size "0", consisting of an orange opaque body and an olive green opaque cap with the imprint "L10" in black ink, filled with a powder that is white to pale yellow in color. Lenalidomide Eugia, 15 mg, hard capsules: [Size approximately 21.4 mm] Hard gelatin capsules, size "0", consisting of a dark orange opaque body and a dark orange opaque cap with the imprint "L15" in black ink, filled with a powder that is white to pale yellow in color. Lenalidomide Eugia, 25 mg, hard capsules: [Size approximately 21.4 mm] Hard gelatin capsules, size "0", consisting of a white opaque body and a white opaque cap with the imprint "L25" in black ink, filled with a powder that is white to pale yellow in color. Pack sizes:7, 14, 21, 28, and 42 hard capsules Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Eugia Pharma (Malta) Ltd. Vault 14, level 2 Valetta Waterfront Floriana, FRN1914 Malta e-mail: [email protected]
Manufacturer/Importer:
APL Swift Services (Malta) Ltd. HF26, Hal Far Industrial Estate, Hal Far Birzebbugia, BBG 3000 Malta Generis Farmacêutica, S.A. Rua João De Deus 19, Venda Nova 2700-487 Amadora Portugal Arrow Génériques 26 Avenue Tony Garnier 69007 Lyon France
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium: Lenalidomide Eugia 2.5 mg/5 mg/7.5 mg/10 mg/15 mg/20 mg/25 mg hard capsules, capsules, Hartkapseln France: Lenalidomide Arrow 2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg & 25 mg, capsules. Germany: Lenalidomide PUREN 2.5 mg/5 mg/7.5 mg/10 mg/15 mg/20 mg/25 mg Hartkapseln Netherlands: Lenalidomide Eugia 2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, 25 mg, hard capsules Poland: Lenalidomide Eugia Portugal: Lenalidomide Generis Spain: Lenalidomide Aurovitas 5 mg/10 mg/15 mg/20 mg/25 mg cápsulas duras EFG
Date of last revision of the leaflet: 08/2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAPL Swift Services (Malta) Ltd. Arrow Generiques Generis Farmaceutica S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Lenalidomide EugiaDosage form: Capsules, 5 mgActive substance: lenalidomidePrescription requiredDosage form: Capsules, 10 mgActive substance: lenalidomidePrescription requiredDosage form: Capsules, 15 mgActive substance: lenalidomidePrescription required
Alternatives to Lenalidomide Eugia in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lenalidomide Eugia in Испания
Alternative to Lenalidomide Eugia in Украина
Online doctors for Lenalidomide Eugia
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lenalidomide Eugia – subject to medical assessment and local rules.






