How to use Ketotifen Stulln
Package Leaflet: Information for the User
Ketotifen Stulln, 0.25 mg/ml, Eye Drops, Solution
Ketotifeni hydrogenofumaras
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Ketotifen Stulln and what is it used for
- 2. Important information before using Ketotifen Stulln
- 3. How to use Ketotifen Stulln
- 4. Possible side effects
- 5. How to store Ketotifen Stulln
- 6. Contents of the package and other information
1. What is Ketotifen Stulln and what is it used for
Ketotifen Stulln contains the active substance ketotifen hydrogenofumarate, which is an antiallergic substance. Ketotifen Stulln is used to treat eye symptoms of hay fever.
2. Important information before using Ketotifen Stulln
When not to use Ketotifen Stulln:
- if you are allergic to ketotifen hydrogenofumarate or any of the other ingredients of this medicine (listed in section 6);
Warnings and precautions
Before starting to use Ketotifen Stulln, discuss it with your doctor or pharmacist.
Children
The safety and efficacy of Ketotifen Stulln in children below 3 years of age have not been established.
Ketotifen Stulln and other medicines
If you need to use another eye medicine at the same time as Ketotifen Stulln, wait at least 5 minutes between putting in the different medicines.
Tell your doctor or pharmacist about all the medicines you are taking, or have recently taken, and about any medicines you plan to take.
This is especially important with medicines used to treat:
- depression, anxiety, and sleep disorders,
- allergies (e.g., antihistamines)
Ketotifen Stulln with alcohol
- the medicine may increase the effects of alcohol
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Ketotifen Stulln can be used during breastfeeding.
Driving and using machines
Ketotifen Stulln may cause blurred vision or drowsiness.
If you experience such effects, do not drive or operate machinery until they have resolved.
3. How to use Ketotifen Stulln
Always use this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
The recommended dose for adults, the elderly, and children (from 3 years of age) is 1 drop into the affected eye(s) twice daily (in the morning and evening).
Use in children under 3 years of age
The safety and efficacy of Ketotifen Stulln in children under 3 years of age have not been established.
Instructions for use
- 1. Wash your hands.
- 2. Remove the protective ring by pulling the protruding strip (Fig. 1).
- 3. Open the bottle by removing the cap (Fig. 2).
- 4. Discard the first drop by turning the bottle upside down and squeezing it once to release one drop.
- 5. Tilt your head back and gently pull the lower eyelid down with your finger to form a pocket between the eyelid and the eye (Fig. 3).
- 6. Turn the bottle upside down. Squeeze the inverted bottle to release a drop into the eye (Fig. 4). Do not touch the eye or eyelid with the dropper tip.
- 7. Close your eyes and press the inner corner of the eye with the tip of one finger for about 1 to 2 minutes. This will prevent the drop from draining through the tear duct into the throat and most of the drop will remain in the eye. If necessary, repeat steps 4 to 7 with the other eye.
- 8. After use, wipe off any excess liquid from the dropper tip. Do not touch the dropper tip to avoid microbial contamination.
- 9. Replace the cap to close the bottle (Fig. 5).
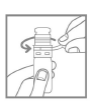
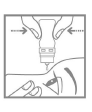
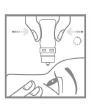
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Using more than the recommended dose of Ketotifen Stulln
There is no risk if you accidentally swallow a few drops of Ketotifen Stulln. Similarly, do not worry if you accidentally put more than one drop into your eye. If you have any doubts, consult your doctor for advice.
Missing a dose of Ketotifen Stulln
If you miss a dose of Ketotifen Stulln, use it as soon as possible, then return to your regular dosing schedule. Make sure the drop gets into your eye. Do not use a double dose to make up for a missed dose.
4. Possible side effects
Like all medicines, Ketotifen Stulln can cause side effects, although not everybody gets them.
The following side effects have been reported:
Common (affects less than 1 in 10 people)
- eye irritation or eye pain,
- eye inflammation,
- corneal damage.
Uncommon (affects less than 1 in 100 people)
- blurred vision after putting in the eye drops,
- dry eye syndrome,
- eyelid irritation,
- conjunctivitis (inflammation of the eye surface),
- increased sensitivity of the eyes to light,
- visible bleeding in the sclera of the eye,
- headache,
- drowsiness,
- rash (which may also be itchy),
- erythema (itchy, red, burning rash),
- dry mouth,
- allergic reaction (including swelling of the face and eyelids) and increased severity of existing allergic conditions, such as asthma or eczema.
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301, Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ketotifen Stulln
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Do not store in the refrigerator or freeze.
Ketotifen Stulln is a sterile medicine without preservatives in a multidose container.
Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiry date refers to the last day of that month.
After first opening the bottle, do not use Ketotifen Stulln for more than 2 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the package and other information
What Ketotifen Stulln contains
- The active substance is ketotifen (as ketotifen hydrogenofumarate). Each ml contains 0.345 mg of ketotifen hydrogenofumarate, equivalent to 0.25 mg of ketotifen.
- The other ingredients are: glycerol, sodium hydroxide 1M (for pH adjustment), and water for injections.
What Ketotifen Stulln looks like and contents of the pack
Ketotifen Stulln is a clear, colorless solution in a transparent bottle.
Ketotifen Stulln is available in packs containing 1 bottle of 10 ml eye drops solution.
Marketing authorization holder and manufacturer
Pharma Stulln GmbH
Werksstrasse 3
92551 Stulln
Germany
For further information about this medicine, please contact the local representative of the marketing authorization holder:
Pharm Supply Sp. z o.o.
ul. Marconich 2/9
02-954 Warsaw
Phone: (+48) 22 6423331
Email: [email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Ketotifen Stulln
Austria
Ketotifen Stulln
Finland
Ketotifen Stulln
Germany
URPEM Multi
Greece
Omnifen
Italy
Ketotifen Stulln
Netherlands
Ketotifen Stulln
Poland
Lidinafree
Portugal
Date of last revision of the package leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterPharma Stulln GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ketotifen StullnDosage form: Drops, 0.25 mg/mlActive substance: ketotifenPrescription not required
Alternatives to Ketotifen Stulln in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ketotifen Stulln in Испания
Alternative to Ketotifen Stulln in Украина
Online doctors for Ketotifen Stulln
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ketotifen Stulln – subject to medical assessment and local rules.