
How to use Izovag
Leaflet attached to the packaging: information for the patient
Izovag, 10 mg/g, vaginal cream
Isoconazole nitrate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist.
- This leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
- If there is no improvement after 7 days or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Izovag and what is it used for
- 2. Important information before using Izovag
- 3. How to use Izovag
- 4. Possible side effects
- 5. How to store Izovag
- 6. Contents of the packaging and other information
1. What is Izovag and what is it used for
Izovag contains the active substance - isoconazole nitrate.
Isoconazole nitrate belongs to antifungal medicines used to treat fungal infections and mixed infections of the vagina, including those involving Gram-positive bacteria.
If there is no improvement after 7 days or the patient feels worse, they should contact their doctor.
2. Important information before using Izovag
When not to use Izovag
- if the patient is allergic to isoconazole nitrate or any of the other ingredients of this medicine (listed in section 6);
- if the patient is a child under 15 years of age, as vaginal applicators are not recommended for this age group.
Warnings and precautions
Before starting to use the vaginal cream Izovag, the patient should discuss it with their doctor or pharmacist.
To treat external genital infections or to prevent infection in the patient's partner, a cream containing isoconazole nitrate, other than the vaginal cream Izovag, is recommended. Izovag cream is not intended for men.
During the 7-day treatment cycle and for one week after its completion, the patient should not use vaginal douching (rinsing).
To prevent recurrence of infections, it is recommended to wear cotton underwear, which should be changed daily to clean, washed, and boiled.
Towels used by the patient should be boiled after each use.
In case of accidental contact of the vaginal cream with the eye, it should be rinsed immediately with a large amount of water.
The ingredients of the medicine may damage latex, reducing the effectiveness of mechanical contraceptive devices (e.g., condoms).
Additional precautions should be taken during the entire treatment period and for 5 days after using the cream.
If there is no improvement after 7 days or the patient feels worse, they should contact their doctor.
Izovag and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
There is no data on the interaction of Izovag with other medicines.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Pregnancy
It is not recommended to use vaginal applicators during pregnancy.
Breastfeeding
Women who are breastfeeding may use Izovag only if, in the doctor's opinion, the benefits of using the medicine for the mother outweigh the potential risks to the child.
Fertility
There is no data indicating that the use of Izovag affects fertility.
Driving and using machines
Izovag has no effect on the ability to drive or operate machinery.
Izovag contains cetostearyl alcohol
The medicine may cause local skin reactions (e.g., contact dermatitis).
3. How to use Izovag
This medicine should always be used as directed by the doctor. If there are any doubts, the patient should consult their doctor or pharmacist.
If there is no improvement after 7 days or the patient feels worse, they should contact their doctor.
Izovag is intended for vaginal use only.
The cream should be used once a day for 7 consecutive days. The packaging contains 7 applicators, each for a single dose.
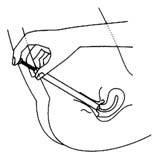
The cream should be inserted into the vagina as deeply as possible. The applicator provided with the packaging is used to apply the cream.
The vaginal cream is recommended to be applied at night, before sleep.
The best position for application is lying on the back.
Treatment should not be continued during menstruation.
Method of use:
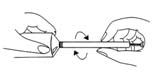
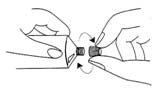
- remove the cap from the tube, pierce the protective membrane, then screw the applicator provided with the packaging into the tube
- pull out the applicator plunger to the end
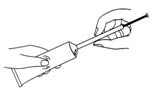
- squeeze the tube to fill the applicator with cream
- remove the applicator and secure the tube with the cap
- insert the applicator deep into the vagina, then press the applicator plunger until all the cream is expelled.
|  |
|  |
|  |
|  |
Using more than the recommended dose of Izovag
A single use of a higher dose of the medicine or even accidental ingestion of the contents of the entire tube does not pose a risk of acute poisoning.
In case of any doubts, the patient should contact their doctor or pharmacist.
Missing a dose of Izovag
A double dose should not be used to make up for a missed dose. If a dose is missed, the next dose should be used as soon as possible, and then treatment should be continued as directed.
Stopping the use of Izovag
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Izovag can cause side effects, although not everybody gets them.
Very rare side effects at the site of application may include: burning and itching.
Allergic reactions such as rash, swelling, nausea, dyspnea, hypotension are also possible.
Reporting side effects
If the patient experiences any side effects, including any not listed in the leaflet, they should inform their doctor or pharmacist, or nurse.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Izovag
There are no special precautions for storing the medicine.
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the packaging after "EXP".
The expiry date refers to the last day of the stated month.
After opening the tube, the cream remains stable for 7 days.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Izovag contains
- The active substance of the medicine is isoconazole nitrate. 1 g of cream contains 10 mg of isoconazole nitrate.
- The other ingredients are: polysorbate 60, sorbitan stearate, cetostearyl alcohol, liquid paraffin, white petrolatum, purified water.
What Izovag looks like and what the packaging contains
Izovag is a white, homogeneous cream.
The packaging of the medicine is an aluminum tube coated with a double layer of epoxy-phenolic varnish with a protective membrane and a HDPE cap, and 7 vaginal applicators made of LDPE with a HDPE plunger, with a capacity of approximately 4.5 g, in a cardboard box.
The tube contains 40 g of vaginal cream.
Marketing authorization holder
Aristo Pharma Sp. z o.o.
ul. Baletowa 30
02-867 Warsaw
+48 22 855 40 93
Manufacturer
Laboratórios Basi - Indústria Farmacêutica, S.A.
Parque Industrial Manuel Lourenço Ferreira, Lotes 8, 15 e 16
3450-232 Mortágua
Portugal
Date of the last update of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterLaboratórios Basi – Indústria Farmaceutica, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IzovagDosage form: Suppositories, 1000 mgActive substance: metronidazoleManufacturer: Dr. August Wolff GmbH & Co. ArzneimittelPrescription requiredDosage form: Suppositories, 1000 mgActive substance: metronidazolePrescription requiredDosage form: Suppositories, 1000 mgActive substance: metronidazolePrescription required
Alternatives to Izovag in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Izovag in Ukraine
Alternative to Izovag in Spain
Online doctors for Izovag
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Izovag – subject to medical assessment and local rules.






