
Ibuvit D3 Babi
Ask a doctor about a prescription for Ibuvit D3 Babi

How to use Ibuvit D3 Babi
Leaflet attached to the packaging: patient information
Ibuvit D Baby, 2 667 IU/ml, oral drops, solution
Cholecalciferol
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
This medicine should always be taken exactly as described in this patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, so you can read it again if you need to.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
- If there is no improvement or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Ibuvit D Baby and what is it used for
- 2. Important information before taking Ibuvit D Baby
- 3. How to take Ibuvit D Baby
- 4. Possible side effects
- 5. How to store Ibuvit D Baby
- 6. Package contents and other information
1. What is Ibuvit D Baby and what is it used for
Ibuvit D Baby contains the active substance vitamin D (cholecalciferol), which plays an important role in bone formation and immune system support. Cholecalciferol is identical to the vitamin D produced in the human body. Vitamin D is found in some foods and is also produced in the body when the skin is exposed to sunlight. Vitamin D increases calcium absorption from the gut and reduces its excretion through the kidneys, which helps build bones. A deficiency of vitamin D can cause rickets (disrupted bone mineralization in children) and weakened immune system. Ibuvit D Baby is used:
- for the prevention of vitamin D deficiency and conditions resulting from vitamin D deficiency (e.g., rickets);
- for the prevention of vitamin D deficiency in women planning pregnancy, during pregnancy, and breastfeeding, in agreement with a doctor.
Preventive intake of vitamin D is recommended during periods of insufficient sun exposure, from October to April or throughout the year, if effective skin synthesis of vitamin D is not ensured during the summer months (see section 1.).
2. Important information before taking Ibuvit D Baby
When not to take Ibuvit D Baby:
- if the patient is allergic to cholecalciferol or any other component of this medicine (listed in section 6);
- if the patient has elevated levels of vitamin D in the blood (hypervitaminosis D);
- if the patient has elevated levels of calcium in the blood (hypercalcemia) or in the urine (hypercalciuria);
- if the patient has severe kidney failure, kidney stones (nephrolithiasis), or a tendency to form kidney stones.
Warnings and precautions
Before starting treatment with Ibuvit D Baby, the patient should discuss it with their doctor or pharmacist:
- if the patient is taking certain heart medications (e.g., cardiac glycosides, such as digoxin) or diuretics from the thiazide group (e.g., hydrochlorothiazide);
- if the patient has sarcoidosis (an immune system disease that can increase vitamin D levels in the body);
- if the patient is taking other medicines and/or dietary supplements containing vitamin D or foods rich in vitamin D, as additional doses of vitamin D can only be taken under medical supervision;
- if the patient is taking additional calcium doses;
- if there is a likelihood that the patient will be exposed to large amounts of sunlight during treatment with Ibuvit D Baby (sufficient sun exposure - see section 1.);
- if the patient has damaged or diseased kidneys;
- if vitamin D treatment is long-term, as the doctor should monitor the patient's calcium levels in the blood and urine, as well as kidney function, by measuring creatinine levels in the blood.
Children
In premature infants, the daily requirement and method of administration of vitamin D should be determined individually by the doctor and verified during regular check-ups, especially in the first months of life. The intake of vitamin D from dietary supplements and foods above a dose of 1000 IU per day carries a risk of vitamin D overdose, especially in newborns with a birth weight below 1000 g.
Ibuvit D Baby and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. This is especially important if the patient is taking:
- medicines that affect the heart or kidneys, such as cardiac glycosides (e.g., digoxin) or diuretics from the thiazide group (e.g., hydrochlorothiazide). Concurrent use with vitamin D may cause a significant increase in calcium levels in the blood and urine;
- medicines containing vitamin D, calcitriol, or other metabolites and analogs of vitamin D, as well as foods rich in vitamin D;
- actinomycin (a medicine used to treat certain types of cancer) and imidazole antifungal medicines (e.g., clotrimazole and ketoconazole, used to treat fungal infections);
- antiepileptic medicines (anticonvulsants);
- barbiturates (sleeping pills, anticonvulsants);
- glucocorticosteroids (steroid hormones, such as hydrocortisone or prednisolone);
- medicines that lower cholesterol levels in the blood (such as cholestyramine or colestipol);
- certain medicines used to treat obesity, which reduce fat absorption (e.g., orlistat);
- certain laxatives (e.g., paraffin oil);
- antacids containing magnesium or aluminum (used for heartburn and indigestion);
- medicines used to treat tuberculosis (e.g., rifampicin, isoniazid).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking this medicine. Ibuvit D Baby should be used during pregnancy and breastfeeding in agreement with a doctor. Administration of vitamin D during pregnancy in a dose of 2000 IU per day has a beneficial effect on pregnant women and their children. During pregnancy, women should follow their doctor's recommendations, as their vitamin D requirements may vary depending on the degree of deficiency and response to treatment.
Driving and operating machinery
Ibuvit D Baby does not affect the ability to drive or operate machinery.
3. How to take Ibuvit D Baby
This medicine should always be taken exactly as described in the patient leaflet or as directed by a doctor or pharmacist. In case of doubt, the patient should consult their doctor or pharmacist. Note: 1 dose (1 drop) contains 400 IU of vitamin D. The abbreviation IU (International Unit) denotes the international units used to measure the activity of vitamin D.
Prevention of vitamin D deficiency and conditions resulting from vitamin D deficiency
In patients with normal body weight, from October to April or throughout the year, if effective skin synthesis of vitamin D is not ensured during the summer months (see section 1.). Premature infants: Ibuvit D Baby should be used in premature infants only under medical supervision and as directed by the doctor. The usual dose is 400-800 IU (1-2 drops) depending on the child's health, weight, and gestational age at birth. The dosage must be determined by the attending physician. Newborns and infants (up to 1 year of age)
- 400 IU (1 drop) Newborns and infants should be given the medicine as directed by the doctor.
Children aged 1 to 3 years
- 400 IU (1 drop)
Children aged 4 to 10 years
- 800 IU (2 drops)
Children with excess weight
Children with excess weight (body mass index (BMI) > 90th percentile for age and sex) require a double dose of vitamin D compared to their peers with normal body weight.
Prevention of vitamin D deficiency in women planning pregnancy, during pregnancy, and breastfeeding
The recommended daily dose is usually 2000 IU (5 drops), regardless of the time of year, unless the doctor recommends otherwise. During pregnancy, women should follow their doctor's recommendations, as their vitamin D requirements may vary depending on their body's vitamin D stores. Ibuvit D Baby should not be taken for longer than recommended or in higher doses, nor should other medicines, dietary supplements, or foods containing vitamin D (cholecalciferol), calcitriol, or other metabolites and analogs of vitamin D be taken without consulting a doctor. The doctor may recommend measuring 25(OH)D levels in the blood. Administration method: Oral. Ibuvit D Baby is best taken during a meal. Ibuvit D Baby should not be mixed with milk in a bottle or with soft foods in containers, as the child may not consume the entire dose. The entire dose of the medicine must be ensured. The solution should not be poured or pumped directly into the mouth from the bottle or dosing pump. The dose of the medicine should be measured using the dosing pump and placed on a spoon.
Instructions for preparing and taking the medicine
Before the first use, the dosing pump must be attached to the bottle. To remove the cap from the bottle, it should be unscrewed in the opposite direction to the clockwise direction, and then removed (fig. 1).
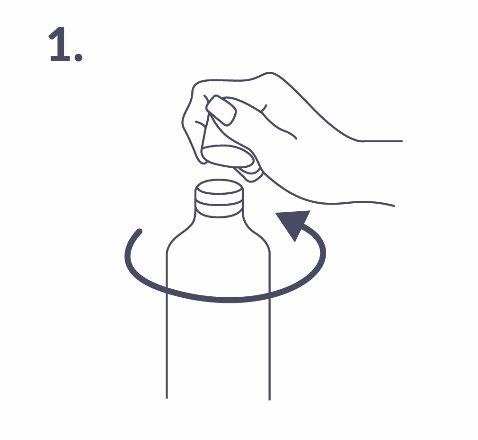
Attaching the dosing pump to the bottle: The dosing pump should be placed on the bottle by carefully inserting the plastic tube into the bottle. Then, the dosing pump should be held on the bottle neck and screwed clockwise until it stops (fig. 2). The dosing pump should be screwed on only once, before starting to use it. It should not be unscrewed and removed later.
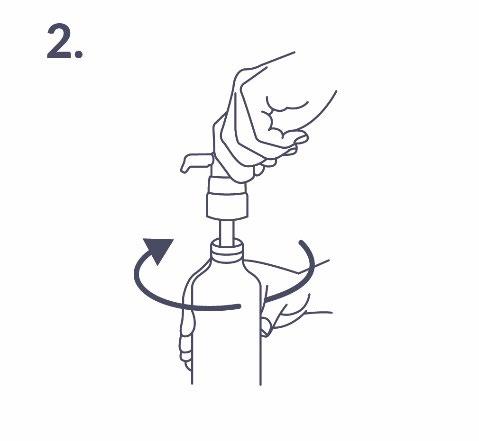
Preparing the dosing pump: When used for the first time, the dosing pump does not dispense the correct amount of solution. Therefore, it should be prepared by pressing the pump head all the way down five times (fig. 3).
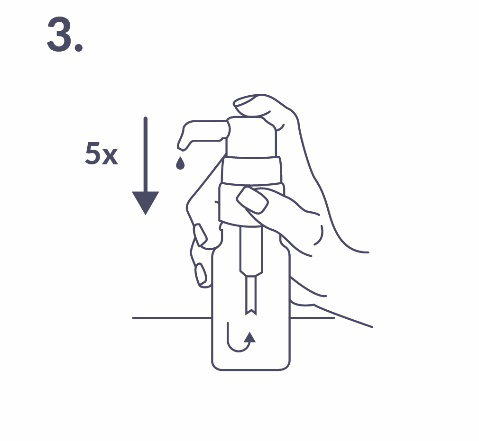
The solution that leaked from the pump should be discarded. At the next full press of the dosing pump (which corresponds to one activation of the pump), the patient will receive the correct dose of the medicine (1 dose, corresponding to one press of the dosing pump, delivers 0.15 ml of solution and contains 400 IU of vitamin D; fig. 4).
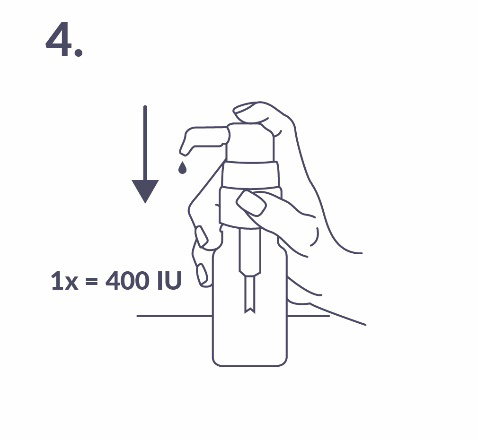
Proper use of the dosing pump: The bottle should be placed on a flat, horizontal surface, such as a table, and used only in an upright position. A spoon should be placed under the nozzle of the dosing pump, and then the pump head should be pressed firmly but calmly (not too slowly) until resistance is felt (fig. 5).
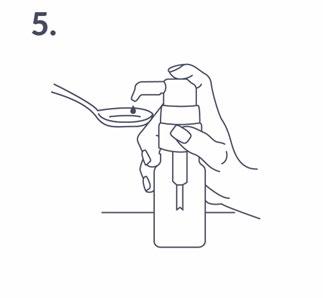
Then, the pump head can be released. After this operation, the pump is ready to measure the next dose of the solution. The dosing pump is intended only for administering the dose of Ibuvit D Baby contained in the supplied bottle. The pump cannot be used to dose other substances or attached to other containers. The bottle with the attached dosing pump should be stored and transported only in an upright position.
Taking a higher dose of Ibuvit D Baby than recommended
If the patient accidentally takes one dose too much, the occurrence of overdose symptoms is unlikely. If the patient has taken too much of the medicine, they should tell their doctor or pharmacist or contact the nearest hospital emergency department for further advice. If possible, they should take the package and this leaflet with them to show the doctor.
Missing a dose of Ibuvit D Baby
A double dose should not be taken to make up for a missed dose. If the patient has any further doubts about taking this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Ibuvit D Baby can cause side effects, although not everybody gets them.
Some side effects may be serious and may require immediate medical attention
Medical attention. The patient should immediately consult their doctor if they experience symptoms of angioedema, such as:
- swelling of the face, tongue, or throat (larynx)
- difficulty swallowing
- hives and difficulty breathing.
Other side effects associated with Ibuvit D Baby include:
Uncommon(occurring in less than 1 in 100 patients)
- elevated calcium levels in the blood (hypercalcemia)
- elevated calcium levels in the urine (hypercalciuria)
Rare(occurring in less than 1 in 1000 patients)
- rash
- itching
- hives
Frequency not known(cannot be estimated from the available data)
- constipation
- gas (bloating)
- nausea
- abdominal pain
- diarrhea.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ibuvit D Baby
The medicine should be stored out of sight and reach of children. Do not store above 25°C. Store in the outer packaging to protect from light. Do not use this medicine after the expiry date stated on the carton and bottle. The expiry date refers to the last day of the month. The opened package should be used within 3 months. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Ibuvit D Baby contains:
- The active substance of the medicine is cholecalciferol. Each drop, which is 1 dose, contains 400 IU of cholecalciferol (vitamin D).
- The other ingredient is: triglycerides of fatty acids with a medium chain length.
What Ibuvit D Baby looks like and what the package contains
Ibuvit D Baby is a clear, colorless to light yellow solution. The bottle is made of orange glass type III, with a PE cap and a dosing pump made of PP/LDPE, in a cardboard box. The package contains 10 ml of solution.
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A., ul. Pelplińska 19, 83-200 Starogard Gdański, tel. +48 22 364 61 01
Manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A., Medana Division in Sieradz, ul. Władysława Łokietka 10, 98-200 Sieradz
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterZakłady Farmaceutyczne POLPHARMA S.A. Oddział Medana w Sieradzu
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ibuvit D3 BabiDosage form: Tablets, 1000 IUActive substance: colecalciferolPrescription requiredDosage form: Tablets, 7000 IUActive substance: colecalciferolPrescription requiredDosage form: Tablets, 30,000 IUActive substance: colecalciferolPrescription required
Alternatives to Ibuvit D3 Babi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ibuvit D3 Babi in Spain
Alternative to Ibuvit D3 Babi in Ukraine
Online doctors for Ibuvit D3 Babi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ibuvit D3 Babi – subject to medical assessment and local rules.














