

Iberogast Balance

Ask a doctor about a prescription for Iberogast Balance

How to use Iberogast Balance
Package Leaflet: Information for the Patient
Iberogast Balance
oral drops, liquid
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
This medicine should always be taken exactly as described in this package leaflet for the patient or as directed by a doctor or pharmacist.
- Keep this package leaflet, so you can read it again if you need to.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any not listed in this package leaflet, they should inform their doctor or pharmacist. See section 4.
- If after 7 days there is no improvement or the patient feels worse, they should contact their doctor.
Package Leaflet Contents
- 1. What is Iberogast Balance and what is it used for
- 2. Important information before taking Iberogast Balance
- 3. How to take Iberogast Balance
- 4. Possible side effects
- 5. How to store Iberogast Balance
- 6. Package contents and other information
1. What is Iberogast Balance and what is it used for
Iberogast Balance is a herbal medicinal product used for the treatment of functional dyspepsia, with main symptoms such as stomach pain, heartburn, feeling full after meals, and early feeling of fullness, but often also loss of appetite, excessive belching, and heartburn. Iberogast Balance is indicated for use in adults over 18 years of age. If after 7 days there is no improvement or the patient feels worse, they should contact their doctor.
2. Important information before taking Iberogast Balance
When not to take Iberogast Balance
If the patient is allergic to any of the active substances (bitter candytuft herb, chamomile flower, caraway fruit, lemon balm leaf, peppermint leaf, or licorice root) or other plants of the Apiaceae or Asteraceae families.
Warnings and precautions
Before taking Iberogast Balance, the patient should discuss it with their doctor or pharmacist. If after 7 days there is no improvement, the patient feels worse, or new symptoms appear, they should consult their doctor to rule out other diseases.
Children and adolescents
The use of Iberogast Balance in children and adolescents under 18 years of age has not been established due to a lack of appropriate data. Therefore, it is not recommended to use the preparation in children and adolescents.
Iberogast Balance and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. No interactions with other medicines have been found.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking this medicine. Data on the use of Iberogast Balance in pregnant women are not available or are limited. As a precaution, it is recommended to avoid using Iberogast Balance during pregnancy. There is insufficient data on the passage of Iberogast Balance active substances or their metabolites into human milk. A risk to the breastfed child cannot be excluded. The patient should consult their doctor.
Driving and using machines
Iberogast Balance has no influence or negligible influence on the ability to drive and use machines.
Iberogast Balance contains ethanol
The medicine contains 240 mg of alcohol (ethanol) in 20 drops. The amount of alcohol in a single dose (20 drops/1 mL) of this medicine is equivalent to less than 7 mL of beer or 3 mL of wine. The small amount of alcohol in this medicine does not have noticeable effects.
3. How to take Iberogast Balance
This medicine should always be taken exactly as described in this package leaflet for the patient or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
Recommended dose
Adults over 18 years of age:
20 drops 3 times a day.
Patients with renal or hepatic impairment:
There is insufficient data on specific dosage recommendations in cases of renal or hepatic impairment.
Method of administration
The medicine should be taken in a small amount of liquid before meals or during meals. Oral administration. Shake before use! When dosing, hold the bottle with the dropper at a 45° angle. After use, close the cap tightly.
Instructions for the first use of the dropper
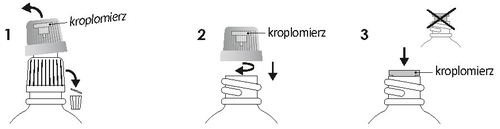
Unscrew the cap and discard the white part. Screw the purple cap tightly to place the dropper in the bottle. Make sure the dropper is well inserted.
Duration of use
If symptoms persist during the use of the medicinal product, the patient should consult their doctor or pharmacist (also see warnings in section 2 Important information before taking Iberogast Balance).
Use in children and adolescents
The use of Iberogast Balance in children and adolescents under 18 years of age has not been established due to a lack of appropriate data. Therefore, it is not recommended to use the preparation in children and adolescents.
Overdose of Iberogast Balance
Accidental ingestion of a higher number of drops than recommended is unlikely to cause adverse effects. In case of ingestion of a significantly higher dose than recommended, the patient should consult their doctor.
Missed dose of Iberogast Balance
The patient should not take a double dose to make up for a missed dose. If the patient has any further doubts about the use of this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Iberogast Balance can cause side effects, although not everybody gets them. Allergic reactions with symptoms such as difficulty breathing or skin reactions, e.g., itching or rash, may occur. Based on available data, the frequency is unknown (cannot be estimated from the available data).
Reporting side effects
If the patient experiences any side effects, including any not listed in this package leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information on the safety of the medicine can be collected.
5. How to store Iberogast Balance
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton and label after "EXP". The expiry date refers to the last day of the month. Do not store above 25°C. Shelf life after first opening the bottle: 8 weeks. Medicines should not be disposed of via wastewater. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Iberogast Balance contains
Active substances:
1 mL contains:
0.15 mL of bitter candytuft herb liquid extract (Iberis amara L., herba) (1:1.5-2.5), extraction solvent: ethanol 50% (V/V)
0.30 mL of chamomile flower liquid extract (Matricaria recutita L., flos) (1:2-4), extraction solvent: ethanol 30% (V/V)
0.20 mL of caraway fruit liquid extract (Carum carvi L., fructus) (1:2.5-3.5), extraction solvent: ethanol 30% (V/V)
0.15 mL of lemon balm leaf liquid extract (Melissa officinalis L., folium) (1:2.5-3.5), extraction solvent: ethanol 30% (V/V)
0.10 mL of peppermint leaf liquid extract (Mentha x piperita L., folium) (1:2.5-3.5), extraction solvent: ethanol 30% (V/V)
0.10 mL of licorice root liquid extract (Glycyrrhiza glabra L. and/or G. inflata Bat. and/or G. uralensis Fisch, radix) (1:2.5-3.5), extraction solvent: ethanol 30% (V/V)
Total ethanol content 31% (V/V).
1 mL = 20 drops
What Iberogast Balance looks like and contents of the pack
Iberogast Balance is a dark brown, clear or slightly cloudy liquid, packaged in orange glass bottles with a closure system containing a dropper and cap. Pack sizes: 20 mL, 50 mL, or 100 mL. Not all pack sizes may be marketed.
Marketing authorization holder
Bayer Sp. z o.o.
Al. Jerozolimskie 158
02-326 Warsaw
Tel.: +48 22 572 35 00
Manufacturer
Steigerwald Arzneimittelwerk GmbH
Havelstraße 5
64295 Darmstadt
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: Iberogast BALANCE Tropfen zum Einnehmen
Bulgaria: Iberogast Balance oral drops, liquid
Croatia: Iberohexa oralne kapi, tekućina
Estonia: Iberoherb
Hungary: Iberogast Select belsőleges oldatos cseppek
Latvia: Iplaceh
Lithuania: Iberoherb geriamasis skystis
Norway: Iberoherb
Poland: Iberogast Balance
Romania: Iberogast Balance picături orale, soluție
Slovakia: Iberogast Neo
Slovenia: Iberohexa peroralne kapljice, raztopina
Sweden: Iberoherb
Date of last revision of the package leaflet:
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterSteigerwald Arzneimittelwerk GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Iberogast BalanceDosage form: Drops, 66.66 mg/mlActive substance: siliconesPrescription not requiredDosage form: Drops, 100 mg/mlActive substance: siliconesManufacturer: Berlin-Chemie AG (Menarini Group)Prescription not requiredDosage form: Drops, 100 mg/mlActive substance: siliconesPrescription not required
Alternatives to Iberogast Balance in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Iberogast Balance in Spain
Alternative to Iberogast Balance in Ukraine
Online doctors for Iberogast Balance
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Iberogast Balance – subject to medical assessment and local rules.














