
Hexvix

Ask a doctor about a prescription for Hexvix

How to use Hexvix
Patient Information Leaflet: User Information
Hexvix, 85 mg, powder and solvent for solution for bladder instillation
Hexyl aminolevulinate hydrochloride
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- Ask your doctor or pharmacist if you need advice or more information.
- If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or nurse. See section 4.
Table of Contents of the Leaflet:
- 1. What is Hexvix and what is it used for
- 2. Important information before using Hexvix
- 3. How to use Hexvix
- 4. Possible side effects
- 5. How to store Hexvix
- 6. Package contents and other information
1. What is Hexvix and what is it used for
Diagnostic agent only.
This medicine is used to detect bladder cancer. The medicine is given before an examination using a device called a cystoscope to view the inside of the bladder. The cystoscope allows you to see possible tumors, which can be removed because they glow in blue light after administration of Hexvix.
2. Important information before using Hexvix
When not to use Hexvix
- if you are allergic (hypersensitive) to the active substance or any of the other ingredients of the preparation, including the ingredients contained in the liquid used to dissolve the Hexvix powder (see section 6 of the leaflet: Other information);
- if you have been diagnosed with porphyria (a rare, inherited blood disease).
Warnings and precautions
Before starting treatment with Hexvix, discuss it with your doctor or nurse:
- if you have a urinary tract infection or a burning sensation when urinating;
- if you have recently undergone BCG treatment (immunotherapy with BCG vaccine for superficial bladder cancer);
- if you have recently undergone bladder surgery. These conditions may cause local reactions in the bladder, which can make it difficult to interpret the results during the examination.
Hexvix and other medicines
Tell your doctor about all the medicines you have taken recently, including those that are available without a prescription.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, or think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Ask your doctor if you can drive or operate machinery after the examination with Hexvix.
3. How to use Hexvix
Hexvix will be prepared and administered by qualified medical personnel.
- Hexvix is usually administered in a hospital.
Your doctor will administer Hexvix as follows:
- 1. A catheter (a thin tube) will be inserted into the bladder.
- 2. The bladder will be emptied through the catheter.
- 3. Hexvix will be administered into the bladder through the catheter.
- 4. Hexvix will remain in the bladder for about 60 minutes.
- 5. Then the bladder will be emptied through the catheter.
- 6. The doctor will use a device called a cystoscope to examine the inside of the bladder.
Using more than the recommended dose of Hexvix
In the event of an extension of the time the product stays in the bladder beyond 60 minutes or an increase in the amount of Hexvix, no adverse reactions are expected. In case of doubt, consult your doctor.
4. Possible side effects
Like all medicines, Hexvix can cause side effects, although not everybody gets them.
There is a risk of side effects related to the examination technique used to examine the bladder (cystoscopy). The use of Hexvix as an auxiliary examination in standard cystoscopy to obtain more accurate diagnostic results is generally well tolerated. If side effects occur, they are usually related to standard examination techniques and are mild and transient. The following side effects may occur after the examination with Hexvix:
Common(may affect up to 1 in 10 people):
- nausea, vomiting,
- diarrhea,
- constipation,
- abdominal cramps or pain,
- painful or difficult urination,
- urinary retention (inability to empty the bladder),
- blood in the urine,
- post-examination pain,
- fever (high temperature).
Uncommon(may affect up to 1 in 100 people):
- headache,
- burning sensation when urinating (due to inflammation or infection of the bladder),
- frequent urination (pollakiuria),
- sepsis (blood infection),
- insomnia (lack of sleep or difficulty falling asleep),
- urethral pain (part of the urinary tract that carries urine out of the body),
- urge to urinate,
- increased white blood cell count, increased bilirubin levels (yellow pigment in bile) or liver enzyme activity - confirmed by blood test results,
- anemia (reduced red blood cell count),
- glans penis inflammation,
- back pain,
- gout,
- rash,
- itching (pruritus).
Unknown(frequency cannot be estimated from the available data)
- anaphylactoid shock (drop in blood pressure, increased heart rate, skin rash).
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Hexvix
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the outer carton.
The expiry date refers to the last day of that month.
Powder and solvent: No special precautions for storage.
Solution (after mixing): Store in a refrigerator (2 °C – 8 °C) for no more than 2 hours.
Medical personnel should ensure that the product is stored properly and not used after the expiry date stated on the outer carton.
6. Package contents and other information
What Hexvix contains
- The active substance of the product is hexyl aminolevulinate
- The liquid used to dissolve the Hexvix powder contains disodium phosphate dihydrate, potassium dihydrogen phosphate, sodium chloride, 1N hydrochloric acid, 1N sodium hydroxide, water for injections
What Hexvix looks like and what the pack contains
- Each pack consists of one vial with white to off-white or light yellow powder, containing 85 mg of the active substance, hexyl aminolevulinate, and an ampoule-syringe containing 50 ml of clear, colorless solvent for dissolving the powder.
Hexvix is dissolved and ready for use. The resulting solution is clear to slightly opalescent, colorless or light yellow.
Add two hours to the current time and record the resulting time and date as the expiry date on the syringe label.
The product is intended for single use. Unused residues should be disposed of. There are no special requirements for disposal.
The product has been shown to maintain its chemical and physical stability for 2 hours at a temperature of 2 °C – 8 °C. From a microbiological point of view, the product should be used immediately. If the product is not used immediately, the user is responsible for the further storage period and conditions before use. The product should be stored in a refrigerator (2 °C – 8 °C) for no more than two hours.
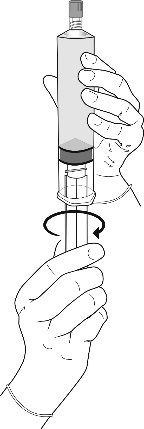
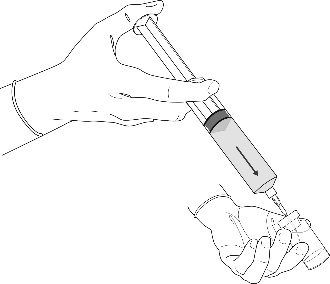
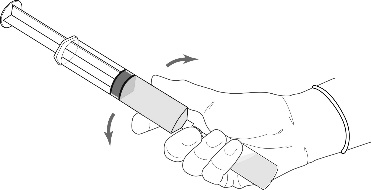
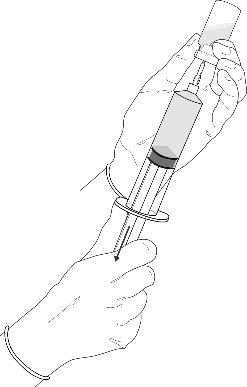
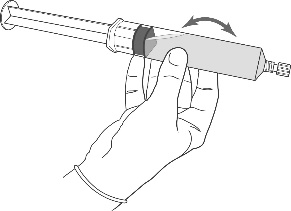
Instructions for preparing the product for use
In case of skin contact, hexyl aminolevulinate may cause sensitization.
All procedures should be performed using sterile equipment and under aseptic conditions.
Preparation procedure: Hexvix powder and solvent for solution in an ampoule-syringe
 |
|
 |
|
 |
|
 |
|
 |
|
Hexvix is dissolved and ready for use. The resulting solution is clear to slightly opalescent, colorless or light yellow.
Add two hours to the current time and record the resulting time and date as the expiry date on the syringe label.
The product is intended for single use. Unused residues should be disposed of. There are no special requirements for disposal.
The product has been shown to maintain its chemical and physical stability for 2 hours at a temperature of 2 °C – 8 °C. From a microbiological point of view, the product should be used immediately. If the product is not used immediately, the user is responsible for the further storage period and conditions before use. The product should be stored in a refrigerator (2 °C – 8 °C) for no more than two hours.
- Country of registration
- Prescription requiredYes
- Manufacturer
- ImporterPhotoCure ASA
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HexvixDosage form: Solution, 40 mg/5 mlActive substance: indigo carmineManufacturer: SERB SERB SAPrescription not requiredDosage form: Capsules, 250 mgActive substance: metyraponePrescription requiredDosage form: Solution, 40 mg/5 mlActive substance: indigo carmineManufacturer: CenexiPrescription not required
Online doctors for Hexvix
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Hexvix – subject to medical assessment and local rules.














