
Haloperidol Vzf

Ask a doctor about a prescription for Haloperidol Vzf

How to use Haloperidol Vzf
Leaflet attached to the packaging: patient information
HALOPERIDOL WZF, 5 mg/ml, solution for injection
Haloperidolum
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Haloperidol WZF and what is it used for
- 2. Important information before using Haloperidol WZF
- 3. How to use Haloperidol WZF
- 4. Possible side effects
- 5. How to store Haloperidol WZF
- 6. Contents of the packaging and other information
1. What is Haloperidol WZF and what is it used for
The name of this medicine is Haloperidol WZF.
The medicine Haloperidol WZF contains the active substance haloperidol. It belongs to a group of medicines called antipsychotics.
Haloperidol WZF is used in adults for diseases that affect thinking, feeling, and behavior. These include mental disorders (such as schizophrenia and bipolar affective disorder) and behavioral disorders.
These diseases can cause the patient to:
- Feel disoriented (confusion)
- See, hear, or smell things that are not there (hallucinations)
- Be convinced of the truth of things that are not true (delusions)
- Experience excessive suspicion (paranoia)
- Experience excitement, agitation, enthusiasm, be impulsive, or overactive
- Be aggressive, hostile, or prone to violence.
Haloperidol WZF is also used in adults:
- to treat uncontrolled movements in Huntington's disease.
- to prevent and treat postoperative nausea and vomiting. Haloperidol WZF may be used as a single medicine or in combination with another medicine. It is sometimes used when other medicines or treatments do not work, cause unacceptable side effects, or cannot be taken orally.
2. Important information before using Haloperidol WZF
When not to use Haloperidol WZF:
- If the patient is allergic to haloperidol or any of the other ingredients of this medicine (listed in section 6).
- If the patient is less aware of their surroundings, or their reactions become unnaturally slow.
- If the patient has Parkinson's disease.
- If the patient has a type of dementia called Lewy body dementia.
- If the patient has progressive supranuclear palsy (PSP).
- If the patient has a condition called QTc interval prolongation, or any other heart rhythm disorders visible in an abnormal ECG (electrocardiogram).
- If the patient has heart failure or has recently had a heart attack.
- If the patient has low potassium levels in the blood that have not been treated.
- If the patient is taking any of the medicines listed in the section "Haloperidol WZF and other medicines", subsection: "Do not use Haloperidol WZF". If any of the above points apply to the patient, they should not take this medicine. In case of doubt, before taking Haloperidol WZF, the patient should consult a doctor, pharmacist, or nurse.
Warnings and precautions
Severe side effects
Haloperidol WZF may cause heart problems, problems with controlling body movements or limbs, and a severe condition called malignant neuroleptic syndrome. It may also cause severe allergic reactions and blood clots. The patient must be aware of the possibility of severe side effects when using Haloperidol WZF, as they may require immediate medical attention. See "Warning about severe side effects" in section 4.
Elderly patients and patients with dementia
In elderly patients with dementia taking antipsychotic medicines, a slight increase in the frequency of deaths and strokes has been observed. If the patient is elderly, especially if they have dementia, they should consult a doctor before receiving Haloperidol WZF.
The patient should consult a doctor or pharmacist if they:
- Have a slow heart rate, heart disease, or if someone in their close family has died suddenly from heart problems.
- Have low blood pressure or feel dizzy when changing position from lying down to sitting or from sitting to standing.
- Have low potassium or magnesium (or other electrolyte) levels in the blood. The treating doctor will decide what treatment to use.
- Have had a stroke in the past or, in the doctor's opinion, are at greater risk of stroke than other patients.
- Have epilepsy or have had seizures in the past.
- Have kidney, liver, or thyroid problems.
- Have high levels of the hormone prolactin in the blood or a tumor that may be caused by high prolactin levels (e.g., breast cancer).
- Have a history of blood clots or have had blood clots in the past.
- Have depression, or if the patient has bipolar affective disorder and depression has started.
It may be necessary to monitor the patient's condition more closely and adjust the dose of Haloperidol WZF.
If the patient has any doubts about whether any of the above points apply to them, they should consult a doctor or nurse before taking Haloperidol WZF.
Control tests
The treating doctor may decide to perform an ECG test before starting or during treatment with Haloperidol WZF. The ECG test measures heart activity.
Blood test
The treating doctor may decide to measure the levels of potassium and magnesium (or other electrolytes) in the blood before starting or during treatment with Haloperidol WZF.
Children and adolescents
Haloperidol WZF should not be used in children and adolescents under 18 years of age. No studies have been conducted in these patient groups.
Haloperidol WZF and other medicines
The patient should tell their doctor, pharmacist, or nurse about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Do not use Haloperidol WZF if the patient is taking certain types of medicines used to treat:
- heart rhythm disorders (such as amiodarone, dofetilide, disopyramide, dronedarone, ibutilide, quinidine, sotalol)
- depression (such as citalopram and escitalopram)
- psychoses (such as fluphenazine, levomepromazine, perphenazine, pimozyd, prochlorperazine, promazine, sertindol, thioridazine, trifluoperazine, triflupromazine, and ziprasidone)
- bacterial infections (such as azithromycin, clarithromycin, erythromycin, levofloxacin, moxifloxacin, and telithromycin)
- fungal infections (such as pentamidine)
- malaria (such as halofantrine)
- nausea and vomiting (such as dolasetron)
- cancer (such as toremifene and vandetanib). The patient should also inform their doctor about taking bepridil (a medicine for chest pain or low blood pressure) or methadone (a pain reliever also used to treat drug addiction). These medicines may increase the risk of heart problems, so the patient should consult a doctor and not use Haloperidol WZF if they are taking any of the listed medicines (see "When not to use Haloperidol WZF").
In case of concurrent use of lithium and Haloperidol WZF, closer monitoring of the patient's condition may be necessary.
The patient should immediately inform their doctor and stop taking both medicines if they experience:
- fever of unknown origin or uncontrolled body movements
- confusion, disorientation, headache, balance problems, drowsiness. These are symptoms of a serious condition.
Certain medicines may affect the way Haloperidol WZF works or may increase the risk of heart problems.
The patient should tell their doctor if they are taking medicines such as:
- alprazolam or buspirone (anxiolytics)
- duloxetine, fluoxetine, fluvoxamine, nefazodone, paroxetine, sertraline, St. John's wort (Hypericum perforatum), or venlafaxine (antidepressants)
- bupropion (for depression or smoking cessation)
- carbamazepine, phenobarbital, or phenytoin (antiepileptics)
- rifampicin (an antibiotic)
- itraconazole, posaconazole, or voriconazole (antifungals)
- ketoconazole (in tablet form, for Cushing's syndrome)
- indinavir, ritonavir, or saquinavir (for HIV infection)
- chlorpromazine or promethazine (for nausea and vomiting)
- verapamil (for high blood pressure or heart disease) The patient should also inform their doctor if they are taking any other medicines to lower blood pressure, such as diuretics. The doctor may need to adjust the dose of Haloperidol WZF if the patient is taking any of the listed medicines.
Haloperidol WZF may affect the way the following types of medicines work.
The patient should tell their doctor if they are taking medicines such as:
- sedatives and sleep aids
- strong pain relievers
- tricyclic antidepressants
- blood pressure lowering medicines (such as guanethidine or methyldopa)
- medicines for severe allergic reactions (such as adrenaline)
- medicines for ADHD or narcolepsy (such as stimulants)
- medicines for Parkinson's disease (such as levodopa)
- blood thinners (such as phenindione). If the patient is taking any of the listed medicines, they should inform their doctor before taking Haloperidol WZF.
Haloperidol WZF and alcohol
Consuming alcohol while using Haloperidol WZF may cause drowsiness and difficulty concentrating. This means that the patient should be careful with the amount of alcohol they consume. The patient should tell their doctor about drinking alcohol while using Haloperidol WZF and how much alcohol they consume.
Pregnancy, breastfeeding, and fertility
Pregnancy-if the patient is pregnant, thinks they may be pregnant, or plans to have a child, they should consult a doctor. The doctor may advise not to use Haloperidol WZF if the patient is pregnant.
In newborns of mothers who used Haloperidol WZF during the last 3 months of pregnancy (last trimester), the following problems may occur:
- muscle tremors, stiffness, or weakness
- excessive drowsiness or agitation
- breathing or feeding problems. The frequency of these disorders is not precisely known. If the patient used Haloperidol WZF during pregnancy and the child experiences any of the listed symptoms, they should contact a doctor. Breastfeeding-The patient should tell their doctor if they are breastfeeding or plan to breastfeed. Small amounts of the medicine may pass into breast milk and then into the child's body. The treating doctor will discuss the risks and benefits of breastfeeding while using Haloperidol WZF. Fertility-Haloperidol WZF may increase the levels of a hormone called prolactin, which can affect fertility in men and women. The patient should consult a doctor if they have any doubts.
Driving and using machines
Haloperidol WZF may affect the patient's ability to drive vehicles, use tools, and operate machines. Side effects such as drowsiness can impair alertness, especially when starting treatment or using a high dose. The patient should not drive vehicles, use tools, or operate machines without first discussing it with their doctor.
3. How to use Haloperidol WZF
What dose will be administered
The treating doctor will decide what dose of Haloperidol WZF the patient needs and for how long. It may take some time before the patient feels the full effect of the medicine. Usually, at the beginning of treatment, the doctor will give the patient a small dose of the medicine, and then adjust the dose according to the patient's needs.
The dose of haloperidol that the patient will receive depends on:
- the patient's age;
- the disease being treated;
- any kidney or liver disease the patient has;
- other medicines the patient is taking.
Adults
- The initial dose is usually 1 to 5 mg.
- The patient may receive additional doses, usually at intervals of 1 to 4 hours.
- The patient will not receive more than 20 mg of the medicine per day.
Elderly patients
- Elderly patients usually start treatment with half of the smallest recommended dose for adults.
- The dose will then be increased until the doctor finds the most suitable dose for the patient.
- The patient will not receive a total daily dose greater than 5 mg, unless the doctor decides that a higher dose is necessary.
How Haloperidol WZF will be administered
Haloperidol WZF will be administered by a doctor or nurse. The medicine is intended for intramuscular use and is administered by injection into a muscle.
Missing a dose or taking more than the recommended dose of Haloperidol WZF
This medicine will be administered to the patient by a doctor or nurse, so missing a dose or overdosing is unlikely. If the patient has any concerns, they should consult a doctor or nurse.
Stopping treatment with Haloperidol WZF
Haloperidol WZF will be discontinued gradually, unless the doctor decides otherwise. Stopping treatment suddenly may cause effects such as:
- nausea and vomiting;
- difficulty sleeping. The patient should strictly follow the doctor's instructions. If the patient has any further doubts about using this medicine, they should consult a doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Haloperidol WZF can cause side effects, although not everybody gets them.
Warning about severe side effects.
If the patient notices or suspects any of the following symptoms, they should immediately inform their doctor. The patient may require urgent medical attention.
Heart problems:
- abnormal heart rhythm - the heart does not work properly, which can cause loss of consciousness
- abnormally fast heart rate
- extra heartbeats. Heart rhythm disorders occur not very often in people taking Haloperidol WZF (may occur in less than 1 in 100 people). There have been cases of sudden deaths in people taking this medicine, but the exact frequency is not known. In people taking antipsychotic medicines, there have also been cases of cardiac arrest (the heart stops beating).
A serious condition called "malignant neuroleptic syndrome". Symptoms include high fever, very strong muscle stiffness, confusion, and loss of consciousness. This condition occurs rarely in people taking Haloperidol WZF (may occur in less than 1 in 1,000 people).
Uncontrolled body movements or limb movements (symptoms of extrapyramidal syndrome), such as:
- movements of the mouth, tongue, jaw, and sometimes limbs (tardive dyskinesia)
- feeling of agitation or difficulty sitting still, increased body movements
- slow or limited body movements, jerky or twisting movements
- muscle tremors, twitching, or uncontrolled muscle contractions, including neck muscle contractions that cause the head to tilt to one side
- difficulty walking
- shortness of breath
- liver inflammation, or liver disorders that cause yellowing of the skin and whites of the eyes (jaundice)
- skin sensitivity to sunlight
- itching
- excessive sweating
- changes in menstrual cycle, such as absence of menstruation, prolonged, heavy, or painful menstruation
- unexpected milk secretion from the breasts
- breast pain or tenderness
- high body temperature
- swelling caused by fluid accumulation in the body. Very common(may occur in more than 1 in 10 people):
- Agitation
- Difficulty sleeping
- Headache.
Common(may occur in less than 1 in 10 people):
- Severe mental disorders, such as delusions or hallucinations
- Depression
- Unnatural muscle tension
- Dizziness, including dizziness when changing position from lying down to sitting or from sitting to standing.
- Drowsiness
- Eye movements upward or rapid, uncontrolled eye movements
- Vision problems, such as blurred vision
- Low blood pressure
- Nausea, vomiting
- Constipation
- Dry mouth or increased saliva production
- Skin rash
- Difficulty urinating or incomplete emptying of the bladder
- Sexual dysfunction (impotence)
- Weight gain or loss
- Changes in liver test results.
Uncommon(may occur in less than 1 in 100 people):
- Affecting blood cells - decreased number of all types of blood cells, including significantly decreased white blood cell count and low platelet count (cells that help blood clot)
- Confusion
- Decreased or lost sexual desire
- Seizures
- Muscle stiffness and joint stiffness
- Muscle spasms, twitching, or uncontrolled muscle contractions, including neck muscle contractions
- Walking problems
- Shortness of breath
- Liver inflammation, or liver disorders that cause yellowing of the skin and whites of the eyes (jaundice)
- Skin sensitivity to sunlight
- Itching
- Excessive sweating
- Changes in menstrual cycle, such as absence of menstruation, prolonged, heavy, or painful menstruation
- Unexpected milk secretion from the breasts
- Breast pain or tenderness
- High body temperature
- Swelling caused by fluid accumulation in the body. Rare(may occur in less than 1 in 1,000 people):
- High levels of the hormone prolactin in the blood
- Narrowing of the airways in the lungs, causing breathing difficulties
- Difficulty opening the mouth or swallowing
- Sexual dysfunction.
The following side effects have also been reported, but their exact frequency is unknown:
- High levels of antidiuretic hormone in the blood (syndrome of inappropriate antidiuretic hormone secretion)
- Low blood sugar
- Swelling around the throat or short-term constriction of the vocal cords, which can cause difficulty speaking and breathing.
- Sudden liver failure
- Decreased bile flow in the bile ducts
- Peeling skin
- Inflammation of small blood vessels, causing a rash of small red or purple spots
- Muscle tissue breakdown (rhabdomyolysis)
- Prolonged and painful erection
- Breast enlargement in men
- Low body temperature.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Medical Devices, and Biocides of the Office for Registration of Medicinal Products, Medical Devices, and Biocides
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Haloperidol WZF
The medicine should be stored out of sight and reach of children.
Store in a temperature below 25°C. Do not freeze. Store in the original packaging to protect from light.
Do not use this medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Haloperidol WZF contains
- The active substance of the medicine is haloperidol. Each ml of the solution contains 5 mg of haloperidol.
- The other ingredients are: lactic acid, water for injections.
What Haloperidol WZF looks like and what the packaging contains
Haloperidol WZF solution for injection is a clear, colorless liquid.
The packaging contains 10 ampoules of 1 ml each, made of colorless glass, in a cardboard box.
Marketing authorization holder and manufacturer
Polpharma S.A.
Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last revision of the leaflet:
---------------------------------------------------------------------------------------------------------------------------
Information intended only for healthcare professionals:
Instructions for opening the ampoule
Before opening the ampoule, make sure that the entire solution is in the lower part of the ampoule.
The ampoule can be gently shaken or tapped with a finger to help the solution flow down.
A colored dot is placed on each ampoule (see Figure 1) as a mark indicating the break point below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing each other - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
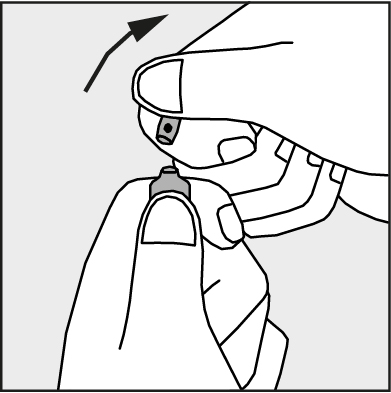
- Press in the direction of the arrow shown in Figure 3. The ampoules are intended for single use only and should be opened just before use. The remaining contents of the unused product should be disposed of in accordance with applicable regulations.
Figure 1.
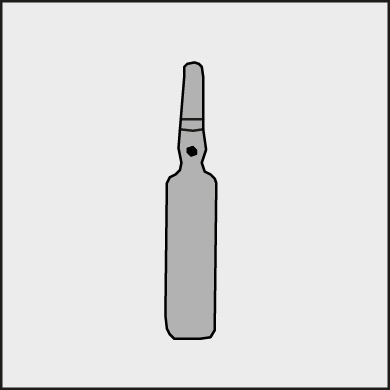
Figure 2.
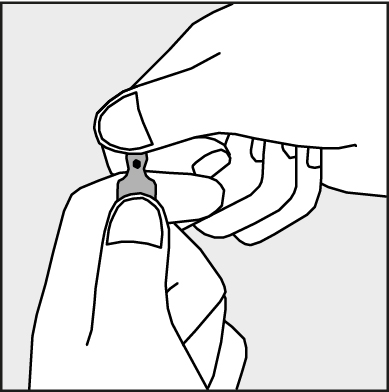
Figure 3.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Haloperidol VzfDosage form: Solution, 50 mg/mlActive substance: haloperidolManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A.Prescription requiredDosage form: Drops, 2 mg/mlActive substance: haloperidolManufacturer: Zakłady Farmaceutyczne "UNIA" Spółdzielnia PracyPrescription requiredDosage form: Drops, 2 mg/mlActive substance: haloperidolPrescription required
Alternatives to Haloperidol Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Haloperidol Vzf in Ukraine
Alternative to Haloperidol Vzf in Spain
Online doctors for Haloperidol Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Haloperidol Vzf – subject to medical assessment and local rules.









