
How to use Ginazol
Package Leaflet: Information for the Patient
GYNAZOL, 20 mg/g, vaginal cream
Butoconazole nitrate
Read the package leaflet carefully before using the medicine, as it contains
important information for the patient.
- This leaflet should be kept in case it needs to be read again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for this person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Gynazol, 20 mg/g, vaginal cream (hereinafter referred to as Gynazol) and what is it used for
- 2. Important information before using Gynazol
- 3. How to use Gynazol
- 4. Possible side effects
- 5. How to store Gynazol
- 6. Package contents and other information
1. What is Gynazol and what is it used for
Gynazol vaginal cream is indicated for the local treatment of vulvovaginal candidiasis caused by Candida albicans.
2. Important information before using Gynazol
When not to use Gynazol
If the patient is allergic to butoconazole or any of the other ingredients of this medicine (listed in section 6).
The efficacy and safety of Gynazol in pregnant women and children have not been established.
Warnings and precautions
Before starting treatment with Gynazol, consult a doctor or pharmacist.
- If the patient's symptoms persist, they should inform their doctor. The doctor may recommend repeating the tests to confirm the initial diagnosis and to rule out other causes that may be causing recurrent fungal infections of the vagina.
- If the patient experiences recurrent vaginal yeast infections, they should inform their doctor, as this may be an early sign of HIV infection, especially in women who are at risk of HIV infection.
- Gynazol contains mineral oil, which may damage products made of rubber or latex, such as condoms or vaginal diaphragms. Therefore, it is not recommended to use these products by the patient or their partner within 72 hours (approximately 3 days) after using Gynazol. If symptoms of allergy or irritation occur during treatment with Gynazol, discontinue use and consult a doctor.
Children and adolescents
The efficacy and safety of Gynazol in children and adolescents have not been established.
Gynazol and other medicines
Inform the doctor or pharmacist about all medicines currently being taken or recently taken, as well as any medicines planned to be taken.
Gynazol contains mineral oil, which may damage products made of latex or rubber, such as condoms or vaginal diaphragms.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
Gynazol can be used during pregnancy only on the advice and under the close supervision of a doctor.
It is not known whether the active substance of Gynazol passes into breast milk. Therefore, the doctor should always be consulted before using this medicine during breastfeeding.
Driving and using machines
Gynazol does not affect the ability to drive or operate machines.
Gynazol contains methyl parahydroxybenzoate, propyl parahydroxybenzoate, and propylene glycol
Propylene glycol may cause skin irritation.
Methyl parahydroxybenzoate and propyl parahydroxybenzoate may cause allergic reactions (possible late-type reactions).
3. How to use Gynazol
This medicine should always be used exactly as advised by the doctor. In case of doubts, consult a doctor or pharmacist.
Gynazol is intended for vaginal use only. The medicine is in a single-dose applicator containing one dose of the medicine.
The entire contents of the applicator should be administered vaginally at one time (see below - Step 3).
The recommended dose is approximately 100 mg of butoconazole nitrate (5 g of cream contained in one applicator). The medicine should be used vaginally once, regardless of the time of day (preferably in the evening).
Step 1 -Preparing the applicator
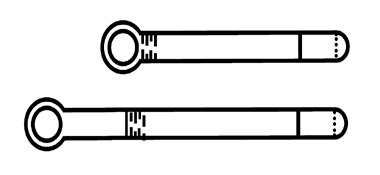
Plunger with ring Tip
Fig. 1 Applicator
- Remove the protective foil and take out the applicator.
- The applicator should be used with the tip originally attached.
- Do not remove the tip.
- Do not use the applicator if the tip has been removed.
- Do not heat the applicator before use.
- Gently holding the applicator, pull the ring until the plunger is fully withdrawn (see: Fig. 1).
Step 2 -Inserting the applicator
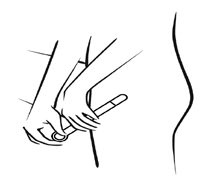
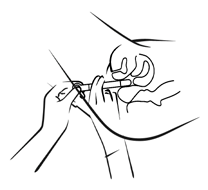
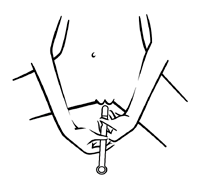
Fig. 2
Fig. 3
Fig. 4
- Carefully insert the applicator into the vagina as far as possible without discomfort (see: Fig. 2, 3).
- To facilitate insertion, this can be done in a lying position (see: Fig. 4). Step 3 -Applying the medicine
- Insert the medicine into the vagina slowly by pressing the plunger.
- After applying the cream, remove the empty applicator from the vagina and then discard it
Using more than the recommended dose of Gynazol
In case of accidental ingestion of Gynazol, consult a doctor or pharmacist as soon as possible, as gastric lavage may be necessary.
In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Gynazol can cause side effects, although not everybody gets them.
The following side effects may occur: burning sensation, itching, pain, and swelling in the vulva or vagina, pain and/or cramps in the lower abdomen. Two or more of these symptoms may occur at the same time.
Local side effects of Gynazol are very similar to the clinical symptoms of vaginal yeast infection or vulvovaginitis. If these symptoms do not disappear during treatment, consult a doctor as soon as possible.
Allergic reactions (hypersensitivity reactions) may occur after using any medicine. Therefore, inform the doctor immediately if any of the following symptoms of allergy occur after using Gynazol: exfoliative dermatitis (redness or peeling), skin or mucous membrane changes, or hives.
Reporting side effects
If any side effects occur, including those not listed in this leaflet, inform the doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Gynazol
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask the pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Gynazol contains
- The active substance is butoconazole nitrate (20 mg in 1 g of cream).
Each filled applicator contains 5 g of vaginal cream, including 100 mg of butoconazole nitrate.
- The other ingredients are: liquid sorbitol, crystallizing; liquid paraffin; glycerol monooleate; polyglyceryl-3-oleate; microcrystalline wax; anhydrous colloidal silica; disodium edetate; methyl parahydroxybenzoate; propyl parahydroxybenzoate; propylene glycol; purified water.
What Gynazol looks like and what the package contains
A homogeneous, gentle cream, white or almost white in color.
Gynazol cream is placed in a polypropylene applicator. Each filled applicator is on a polystyrene tray placed in a laminated bag.
The bag is packed in a cardboard box.
Marketing authorization holder and manufacturer
Gedeon Richter Plc.
Gyömrői út 19-21
1103 Budapest
Hungary
To obtain more detailed information, contact the representative of the marketing authorization holder:
GEDEON RICHTER POLSKA Sp. z o.o.
Medical Department
ul. ks. J. Poniatowskiego 5
05-825 Grodzisk Mazowiecki
phone: +48 22 755 96 48
[email protected]
fax: +48 22 755 96 24
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGedeon Richter Plc.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GinazolDosage form: Suppositories, 1000 mgActive substance: metronidazoleManufacturer: Dr. August Wolff GmbH & Co. ArzneimittelPrescription requiredDosage form: Suppositories, 1000 mgActive substance: metronidazolePrescription requiredDosage form: Suppositories, 1000 mgActive substance: metronidazolePrescription required
Alternatives to Ginazol in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ginazol in Ukraine
Alternative to Ginazol in Spain
Online doctors for Ginazol
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ginazol – subject to medical assessment and local rules.







