
Gexiro
Ask a doctor about a prescription for Gexiro

How to use Gexiro
Leaflet accompanying the packaging: information for the user
Gexiro, 32 mg/mL + 9.6 mg/mL, oral suspension
Paracetamol + Ibuprofen
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for a specific person. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Gexiro and what is it used for
- 2. Important information before taking Gexiro
- 3. How to take Gexiro
- 4. Possible side effects
- 5. How to store Gexiro
- 6. Contents of the pack and other information
1. What is Gexiro and what is it used for
Gexiro contains paracetamol and ibuprofen.
Paracetamol is an active substance that reduces pain.
Ibuprofen belongs to a group of medicines called nonsteroidal anti-inflammatory drugs (NSAIDs).
It reduces pain and inflammation (swelling, redness, or tenderness).
Gexiro is used for the short-term treatment of acute pain of mild to moderate severity, which is believed not to respond to paracetamol or ibuprofen (given separately) in children aged 2 to 12 years and weighing 12 kg or more.
If you have any questions about this medicine, ask your doctor or pharmacist.
2. Important information before taking Gexiro
When not to take Gexiro:
- -if the child is allergic to paracetamol, ibuprofen, or any of the other ingredients of this medicine (listed in section 6);
- if the child has a stomach ulcer (i.e., a stomach or duodenal ulcer), bleeding, or has had two or more episodes of ulceration or bleeding from the stomach or small intestine;
- if the child has ever had bleeding or perforation of the stomach or intestines after treatment with nonsteroidal anti-inflammatory drugs (NSAIDs);
- with other medicines containing paracetamol or ibuprofen;
- if the child has severe heart, liver, or kidney failure;
- if the child has bleeding from blood vessels in the brain or other active bleeding;
- if the child has blood disorders, bleeding disorders, and other diseases that increase the tendency to bleed;
- if the child has ever had asthma, hives, or allergic reactions after taking acetylsalicylic acid or other NSAIDs;
- if the child is severely dehydrated (due to vomiting, diarrhea, or insufficient fluid intake);
- with other NSAIDs (including selective COX-2 inhibitors) or with acetylsalicylic acid in doses greater than 75 mg per day.
In adults, in addition to the above situations, Gexiro should not be taken:
- in women during the last three months of pregnancy.
Warnings and precautions
You should talk to your doctor or pharmacist:
- before starting to give Gexiro to your child;
- if your child has an infection - see below, "Infections".
Warning:Taking a dose higher than recommended increases the risk of severe liver damage.
Therefore, do notexceed the maximum daily dose of paracetamol. Check if
other medicines taken by your child, including those obtained without a prescription, do not contain
paracetamol. Do not combine them to avoid exceeding the recommended daily dose (see section 3
"How to take Gexiro" and "Taking a higher dose of Gexiro than recommended").
You should also check if other medicines taken by your child do not contain ibuprofen.
Taking anti-inflammatory and/or pain-relieving medicines, such as ibuprofen, may slightly increase the risk of heart attack or stroke, especially when taken in high doses. Do not exceed the recommended dose or duration of treatment.
If your child develops severe diseases during treatment with Gexiro, including severe kidney or liver dysfunction, sepsis (when bacteria and their toxins circulate in the blood, leading to organ damage), or malnutrition, alcoholism in the chronic phase, or if your child is also taking flucloxacillin (an antibiotic). Severe metabolic acidosis (a blood and fluid disorder) has been reported in patients taking paracetamol regularly for a long time or taking paracetamol with flucloxacillin.
Symptoms of metabolic acidosis may include: severe breathing difficulties, including rapid deep breathing, drowsiness, nausea (nausea) and vomiting.
In children with dehydration, there is a risk of kidney damage.
Before starting Gexiro, discuss this with your doctor or pharmacist if your child:
- has high blood pressure;
- has high cholesterol levels, or if there is a history of heart disease or stroke in the family;
- has or has had asthma or diabetes;
- has or has had lupus or mixed connective tissue disease;
- has or has had chronic inflammatory bowel disease, such as ulcerative colitis or Crohn's disease with gastrointestinal bleeding;
- has or has had chickenpox;
- has liver disease, hepatitis, kidney disease, or difficulty urinating;
- if surgery is planned for the child;
- has or has had other diseases, including: heartburn, indigestion, stomach ulcers, or any other stomach disorders; bleeding vomiting or bleeding from the rectum; glucose-6-phosphate dehydrogenase enzyme deficiency; hemolytic anemia (a blood disorder); severe skin reactions, such as Stevens-Johnson syndrome;
vision disorders;
swelling of the feet or ankles;
diarrhea;
inherited or acquired disorders of certain enzymes, the symptoms of which are neurological or skin disorders, and sometimes both types of disorders, e.g., porphyria.
This medicine is intended for children aged 2 to 12 years. In adults taking this medicine, all of the above statements apply, as well as the following additional warnings:
Tell your doctor or pharmacist if:
- the patient regularly consumes large amounts of alcohol or other medicines;
- the patient has heart problems, including heart failure, angina (chest pain), or has had a heart attack, bypass surgery, peripheral artery disease (poor circulation in the legs or feet due to narrow or blocked arteries), or has had any stroke [including "mini-stroke" or transient ischemic attack (TIA)];
- the patient is a smoker;
- the patient is pregnant or plans to become pregnant;
- the patient is breastfeeding or plans to breastfeed.
This medicine belongs to a group of medicines (NSAIDs) that may affect fertility in women. This effect is reversible after stopping the medicine.
Skin reactions
Severe skin reactions have been reported with Gexiro. Stop giving Gexiro to your child and seek medical help immediately if your child develops a skin rash, mucosal damage, blisters, or other signs of an allergic reaction, as these may be the first signs of a very severe skin reaction. See section 4.
Infections
Gexiro may mask the symptoms of an infection, such as fever and pain. It is possible that taking Gexiro may delay the start of proper treatment of the infection, which may lead to an increased risk of complications. This has been observed in cases of bacterial pneumonia and bacterial skin infection in chickenpox. If your child has an infection and is taking this medicine, and the symptoms of the infection persist or worsen, you should consult your doctor immediately.
Gexiro and other medicines
Do not take this medicine:
- with other medicines containing paracetamol and ibuprofen;
- with other NSAIDs (including selective COX-2 inhibitors) or with acetylsalicylic acid in doses greater than 75 mg per day.
Tell your doctor or pharmacist about all medicines your child is taking or has recently taken, as well as any medicines your child plans to take.
Gexiro may affect other medicines or other medicines may affect Gexiro. For example:
- anticoagulant medicines (i.e., blood thinners and/or medicines that prevent blood clots, such as acetylsalicylic acid, warfarin, ticlopidine);
- medicines used to treat epilepsy or seizures;
- chloramphenicol, an antibiotic used to treat ear and eye infections;
- probenecid, a medicine used to treat gout;
- zydovudine, a medicine used to treat HIV infection (the virus that causes AIDS);
- medicines used to treat tuberculosis, such as isoniazid and rifampicin;
- acetylsalicylic acid, salicylates, or other NSAIDs;
- medicines that lower high blood pressure (angiotensin-converting enzyme inhibitors, such as captopril, beta-blockers, such as atenolol, angiotensin II receptor antagonists, such as losartan);
- diuretics, also known as water pills;
- lithium, a medicine used to treat certain types of depression;
- methotrexate, a medicine used to treat arthritis and certain types of cancer;
- corticosteroids, such as prednisone, cortisone;
- metoclopramide, domperidone, propantheline;
- cholestyramine, a medicine used to reduce elevated blood fat levels;
- tacrolimus or cyclosporin, immunosuppressive medicines used after organ transplantation;
- sulfonylurea derivatives, medicines used to treat diabetes;
- certain antibiotics (e.g., quinolone antibiotics);
- flucloxacillin (an antibiotic), due to the serious risk of a blood and fluid disorder (called metabolic acidosis), which requires emergency treatment (see section 2);
- aminoglycosides;
- phenytoin;
- CYP2C9 inhibitors, such as voriconazole and fluconazole;
- mifepristone;
- ritonavir;
- selective serotonin reuptake inhibitors (SSRIs), digitalis glycosides, hawthorn, bisphosphonates, and oxpentifylline (pentoxifylline).
Gexiro may affect these medicines or these medicines may affect the effectiveness of Gexiro. Your child may need to take different doses of these medicines or different medicines.
Some other medicines may also affect Gexiro or Gexiro may affect their action. Therefore, before taking Gexiro with other medicines, you should always consult your doctor or pharmacist.
More information about these and other medicines that should be used with caution or avoided during treatment with this medicine can be obtained from your doctor and pharmacist.
Gexiro and alcohol
Do not drink alcoholic beverages while taking this medicine. Consuming alcohol while taking Gexiro may cause liver damage.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before taking this medicine.
Do not take Gexiro during the last 3 months of pregnancy, as it may harm the unborn child or cause problems during delivery. This medicine may cause kidney and heart problems in the unborn child. It may also increase the risk of bleeding in the mother and child and prolong labor.
Do not take Gexiro during the first 6 months of pregnancy, unless your doctor considers it absolutely necessary. If treatment is necessary during this period or when trying to conceive, use the smallest dose for the shortest possible time. From the 20th week of pregnancy, Gexiro may cause kidney problems in the unborn child if taken for more than a few days. This may lead to a decrease in the amount of amniotic fluid surrounding the child (oligohydramnios) or narrowing of the arterial duct (ductus arteriosus) in the child's heart. If treatment is required for a longer period, your doctor may recommend additional monitoring.
This medicine may affect fertility in women and is not recommended for women trying to conceive.
Driving and using machines
Be careful when driving or operating machinery until you know how Gexiro affects you. If you experience symptoms such as dizziness, drowsiness, fatigue, and vision disturbances after taking this medicine, do not drive or operate machinery.
Gexiro contains maltitol liquid, sodium benzoate, sodium, and propylene glycol
- Maltitol liquid: Gexiro contains 250 mg/mL of maltitol liquid. If your child has previously been diagnosed with intolerance to some sugars, consult your doctor before giving this medicine to your child.
- Sodium benzoate: this medicine contains 1 mg of sodium benzoate per mL of suspension.
- Sodium: Gexiro contains less than 1 mmol (23 mg) of sodium per dose, which means the medicine is considered "sodium-free".
- Propylene glycol: this medicine contains 9.6 mg of propylene glycol per mL of suspension, which corresponds to up to 16 mg/kg body weight per day.
3. How to take Gexiro
This medicine should always be taken exactly as advised by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
It is important for children under 10 years of age to follow the dosage based on body weight, not approximate age, which is given for informational purposes only.
In children over 10 years of age, the relationship between body weight and age is no longer uniform due to puberty, which affects body weight differently depending on sex and individual characteristics of the child.
This medicine is not intended for children under 2 years of age and children weighing less than 12 kg.
Doses should be given every 6 hours as needed. Do not give more than 4 doses in 24 hours.
| Body weight | Age (approximate) | Dose (mL) | Maximum daily dose (mL) |
| 2 years | 4.5 | 18 |
| 3 years | 5.5 | 22 |
| 4 years | 6 | 24 |
| 5 years | 7 | 28 |
| 6 years | 7.5 | 30 |
| 7 years | 8.5 | 34 |
| 8 years | 9.5 | 38 |
| 9 years | 10.5 | 42 |
| 10 years | 11.5 | 46 |
| 11-12 years | 12.5 | 50 |
Adults taking this medicine should consult their doctor to determine the dose to take.
Do not take for more than 3 days.
Use the smallest effective dose for the shortest time necessary to relieve symptoms. If your child has an infection, consult your doctor immediately if symptoms (such as pain) persist or worsen.
If your doctor recommends a different dose, follow their advice.
Instructions for using the syringe:
- 1. Shake the bottle for at least 10 seconds before use.
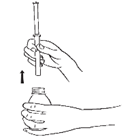
- 2. Press the syringe firmly into the adapter (opening) in the neck of the bottle.
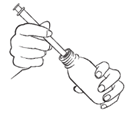
- 3. To fill the syringe, turn the bottle upside down. Holding the syringe still, carefully push the plunger down to draw the medicine into the syringe to the correct mark on the syringe.
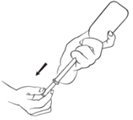
- 4. Turn the bottle right side up and carefully unscrew the syringe from the adapter on the bottle.
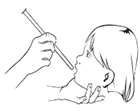
- 5. Place the tip of the syringe in the child's mouth, usually in the corner of the mouth, between the gums and cheek. Slowly press the plunger to empty the syringe.
- 6. If the table above recommends giving more than 5 mL of medicine, repeat the steps in points 2 to 5 to give the correct amount of medicine.
- 7. After use, close the bottle by screwing the cap tightly. All medicines should be kept out of sight and reach of children.
- 8. Wash the syringe with warm water and let it dry.
Do not mix this medicine with food or other liquids.
Taking a higher dose of Gexiro than recommended
If the patient or their child takes a higher dose of this medicine than recommended or if the medicine is accidentally ingested by a child, they should immediately contact a doctor or go to the emergency department of the nearest hospital to get an opinion on the risk and advice on what to do.
This should be done because too high a dose of paracetamol may cause delayed, severe liver damage. This should be done even if the patient does not feel unwell or poisoned. The child may need emergency medical attention.
Other symptoms of overdose may include: nausea, abdominal pain, vomiting (which may contain blood), gastrointestinal bleeding (see also section 4 below), diarrhea, headache, ringing in the ears, confusion, and tremors. There may also be agitation, drowsiness, disorientation, or coma. In individual cases, the patient may experience seizures. After taking large doses, drowsiness, chest pain, palpitations, loss of consciousness, seizures (mainly in children), weakness, and dizziness, blood in the urine, low potassium levels in the blood, feeling cold, and breathing problems have been reported. Additionally, the prothrombin time/INR may be prolonged, probably due to disorders of circulating clotting factors in the blood. Acute kidney failure and liver damage may occur. In people with asthma, asthma may worsen. Additionally, low blood pressure and decreased respiratory rate may occur.
Missing a dose of Gexiro
If it is almost time for the next dose, skip the missed dose and take the next dose as planned. Otherwise, take the medicine as soon as you remember, and then return to the usual dosing schedule of the suspension.
Do not take a double dose to make up for a missed dose.
If you are unsure whether to skip a missed dose, talk to your doctor or pharmacist.
4. Possible side effects
Like all medicines, Gexiro can cause side effects, although not everybody gets them.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
If you experience any of the following serious side effects, stop taking Gexiro and contact your doctor or go to the emergency department of the nearest hospital immediately:
Uncommon (may affect up to 1 in 100 people):
- vomiting blood or coffee grounds;
- bleeding from the rectum, black, tarry stools, or bloody diarrhea;
- swelling of the face, lips, or tongue, which may cause difficulty swallowing or breathing.
Rare (may affect up to 1 in 1,000 people):
- hives.
Very rare (may affect up to 1 in 10,000 people):
- asthma, wheezing, shortness of breath;
- sudden or severe itching, rash;
- severe rash with blisters and bleeding in the mouth, eyes, nose, and genitals (Stevens-Johnson syndrome). Very rare cases of severe skin reactions have been reported;
- worsening of existing severe skin infections (may cause rash, blisters, and discoloration of the skin, fever, drowsiness, diarrhea, and nausea) or worsening of other infections, including chickenpox or shingles or severe infection with tissue destruction (necrosis) and blistering and peeling of the skin;
- fever;
- general malaise;
- stiff neck.
Other side effects that may occur:
Common (may affect up to 1 in 10 people):
- nausea or vomiting;
- loss of appetite;
- heartburn or stomach pain;
- stomach cramps, gas, constipation, or diarrhea, minor gastrointestinal bleeding;
- skin rash, itching;
- headache;
- dizziness;
- feeling agitated;
- ringing or buzzing in the ears;
- unusual weight gain, swelling, and fluid retention, swelling of the feet or ankles;
- increased alanine aminotransferase activity, increased gamma-glutamyltransferase activity, and abnormal liver function test results after taking paracetamol, increased creatinine and urea levels in the blood.
Uncommon (may affect up to 1 in 100 people):
- decreased red blood cell count, nosebleeds, and heavy menstrual bleeding (menstrual bleeding);
- allergic reactions - skin rash, fatigue, joint pain (e.g., rheumatic disease, lupus, Henoch-Schönlein purpura, angioedema);
- breast enlargement in men;
- low blood sugar levels;
- insomnia;
- mood changes, such as depression, disorientation;
- eye problems, such as blurred vision (reversible), eye pain, and redness;
- thickening of mucus in the airways;
- wheezing due to airway obstruction in children during tonsillectomy;
- low oxygen levels in the blood;
- severe stomach pain or tenderness; stomach ulcer and/or gastrointestinal perforation;
- inflammation of the intestines and worsening of inflammatory bowel disease (inflammatory bowel disease) and gastrointestinal complications (perforation or fistula);
- inability to fully empty the bladder (urinary retention);
- abnormal laboratory test results (blood, liver, and kidney enzymes);
- postoperative bleeding after tonsillectomy;
- ulcerative stomatitis.
Rare (may affect up to 1 in 1,000 people):
- tingling in the hands and feet;
- unusual dreams, seeing things that are not there (hallucinations);
- kidney tissue damage (especially with long-term use of the medicine);
- high uric acid levels in the blood (hyperuricemia).
Very rare (may affect up to 1 in 10,000 people):
- low potassium levels - weakness, fatigue, muscle cramps (hypokalemia);
- symptoms of anemia, such as fatigue, headaches, shortness of breath, and pale skin;
- easy bruising or bleeding, or red or purple spots under the skin;
- severe or persistent headache;
- feeling dizzy;
- fast or irregular heartbeat, also known as palpitations;
- increased blood pressure and possible heart problems;
- esophageal inflammation;
- yellowing of the skin and/or eyes, also known as jaundice;
- liver damage (especially with long-term use of the medicine);
- hair loss;
- increased sweating;
- symptoms of frequent or worrying infections, such as fever, chills, sore throat, or mouth ulcers;
- cloudy urine;
- skin sensitivity to light;
- effects of the medicine opposite to those expected, pain when moving the eyes or transient loss of vision, gait disturbances or involuntary movements, tremors, or seizures;
- abdominal pain, bloating, or gas retention and constipation;
- red or purple rash.
Frequency not known (frequency cannot be estimated from the available data):
- a severe skin reaction known as DRESS syndrome may occur. The symptoms of DRESS syndrome include: skin rash, fever, swelling of the lymph nodes, and an increased number of eosinophils (a type of white blood cell);
- a red, scaly, widespread rash with bumps and blisters, mainly on the folds of the skin, torso, and upper limbs, with fever in the early stages of treatment (acute generalized exanthematous pustulosis). If you experience such symptoms, stop taking Gexiro and seek medical help immediately. See also section 2.
- a serious condition that can make the blood more acidic (metabolic acidosis), in patients with severe illness taking paracetamol (see section 2).
The above list includes serious side effects that may require medical attention.
Serious side effects are rare after taking small doses of this medicine and when it is used for a short period.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Gexiro
Keep the medicine out of sight and reach of children.
Store at a temperature below 25°C.
Shelf life after first opening: 3 months when stored at 25°C or below.
Do not use this medicine after the expiry date stated on the carton and bottle after "Expiry date (EXP)". The expiry date refers to the last day of the month stated.
Do not use this medicine if you notice any damage to the packaging or if the packaging shows signs of tampering.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Gexiro contains
The active substances of Gexiro are: paracetamol 32 mg and ibuprofen 9.6 mg per 1 mL of suspension.
The other ingredients are: citric acid monohydrate (E 330), glycerol (E 422), maltitol liquid (E 965),
polysorbate 80, sodium benzoate (E 211), sodium citrate (E 331), sucralose (E 955), microcrystalline cellulose and sodium carmellose (Vivapur MCG 591P), xanthan gum, fruit flavor, masking bitter taste*, strawberry flavor*, candy flavor*, vanilla flavor*, carmine (E 120), purified water.
* Contains propylene glycol (E 1520)
What Gexiro looks like and contents of the pack
Gexiro is a viscous oral suspension, pink in color.
The pack contains 100 mL or 200 mL of suspension in a PET bottle with a PP/HDPE cap with a child-resistant closure and an oral syringe with a capacity of 5 mL (with a scale every 0.1 mL), which serves as a dosing device.
Marketing authorization holder and manufacturer
Marketing authorization holder
Medical Valley Invest AB
Brädgårdsvägen 28
236 32 Höllviken
Sweden
email: [email protected]
Manufacturer
SAG Manufacturing S.L.U.
Carretera N-I, Km 36, San Agustin de Guadalix
28750 Madrid
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Greece:
GoPain Oral Suspension
Malta:
Paraibucomb
Poland:
Gexiro
Date of last revision of the leaflet:01/2025
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSAG MANUFACTURING, S.L.U.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GexiroDosage form: Tablets, 325 mg + 30 mg + 10 mgActive substance: paracetamol, combinations excl. psycholepticsManufacturer: Polfarmex S.A.Prescription not requiredDosage form: Granulate, 500 mg + 200 mg + 4 mgActive substance: paracetamol, combinations excl. psycholepticsManufacturer: Natur Produkt Pharma Sp. z o.o.Prescription not requiredDosage form: Tablets, 500 mg + 200 mg + 4 mgActive substance: paracetamol, combinations excl. psycholepticsManufacturer: Natur Produkt Pharma Sp. z o.o.Prescription not required
Alternatives to Gexiro in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Gexiro in Spain
Alternative to Gexiro in Ukraine
Online doctors for Gexiro
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Gexiro – subject to medical assessment and local rules.














