

Formetic

Ask a doctor about a prescription for Formetic

How to use Formetic
Leaflet accompanying the packaging: patient information
Formetic
850 mg, coated tablets
Metformin hydrochloride
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Formetic and what is it used for
- 2. Important information before taking Formetic
- 3. How to take Formetic
- 4. Possible side effects
- 5. How to store Formetic
- 6. Contents of the pack and other information
1. What is Formetic and what is it used for
Formetic belongs to a group of medicines used to treat non-insulin-dependent diabetes (type 2 diabetes) in adults and children over 10 years old.
Formetic is a medicine that lowers blood sugar levels in patients with diabetes (type 2 diabetes),
especially in patients who are overweight and cannot achieve proper blood sugar levels through diet and physical activity.
- 2); especially in patients who are overweight and cannot achieve proper blood sugar levels through diet and physical activity.
Adults
In adults, the doctor may prescribe Formetic as the only anti-diabetic medicine (monotherapy) or in combination with other oral anti-diabetic medicines or insulin.
Children and adolescents
In children over 10 years old and adolescents, the doctor may prescribe Formetic as the only anti-diabetic medicine (monotherapy) or in combination with insulin.
It has been shown that the complications of type 2 diabetes can be reduced in patients with type 2 diabetes and overweight who are treated with metformin as the first-line treatment, in whom dietary treatment has not produced the expected results.
Formetic can be used in a condition called pre-diabetic state, when proper blood sugar control cannot be achieved through diet and physical activity.
2. Important information before taking Formetic
When not to take Formetic
- if the patient is allergic to metformin or any of the other ingredients of this medicine (listed in section 6),
- if the patient has a pre-coma state in diabetes,
- if the patient has significantly reduced kidney function,
- if the patient has uncontrolled diabetes, such as severe hyperglycemia (high blood glucose levels), nausea, vomiting, diarrhea, sudden weight loss, lactic acidosis (see "Risk of lactic acidosis" below) or ketoacidosis. Ketoacidosis is a disease in which substances called ketone bodies accumulate in the blood and can lead to a diabetic pre-coma state. Symptoms include: abdominal pain, rapid and deep breathing, drowsiness or a characteristic fruity odor from the mouth.
- if the patient has kidney function impairment due to
- dehydration caused by prolonged vomiting or severe diarrhea
- severe infection
- shock (shock)
- if the patient has acute or chronic diseases that may cause tissue hypoxia (tissue hypoxia), such as:
- heart or respiratory failure
- recent myocardial infarction
- shock (shock)
- if the patient has liver failure, acute alcohol poisoning, or alcoholism.
Warnings and precautions
Before starting treatment with Formetic, the patient should discuss it with their doctor or pharmacist.
Risk of lactic acidosis
Formetic may rarely cause a very serious side effect called lactic acidosis, especially if the patient has kidney problems. The risk of lactic acidosis increases in case of uncontrolled diabetes, severe infection, prolonged fasting, or alcohol consumption, dehydration (see more information below), liver dysfunction, and any conditions in which a part of the body is not sufficiently supplied with oxygen (e.g., severe heart disease).
If any of the above circumstances apply to the patient, they should consult their doctor for more detailed instructions.
The patient should temporarily stop taking Formetic if they have a condition that may be associated with dehydration
(significant loss of body water), such as severe vomiting, diarrhea, fever, exposure to high temperatures, or if the patient drinks less fluid than usual. The patient should consult their doctor for more detailed instructions.
The patient should stop taking Formetic and immediately contact their doctor or the nearest hospital if they experience any symptoms of lactic acidosis
, as this condition can lead to coma.
Symptoms of lactic acidosis include:
- vomiting,
- abdominal pain,
- muscle cramps,
- general feeling of being unwell with severe fatigue,
- breathing difficulties,
- decreased body temperature and slowed heart rate.
Lactic acidosis is a life-threatening condition that requires immediate hospital treatment.
The patient should immediately consult their doctor for further instructions if:
- they have a genetically inherited disease affecting the mitochondria (energy-producing structures in cells), such as MELAS syndrome (mitochondrial encephalopathy, myopathy, lactic acidosis, and stroke-like episodes) or
maternally inherited diabetes and deafness (MIDD).
- after starting metformin, the patient experiences any of the following symptoms: seizures, impaired cognitive function, difficulty moving, signs of nerve damage (e.g., pain or numbness), migraine, and hearing loss.
If the patient is to undergo major surgery, they should not take Formetic during or after the surgery for a certain period. The doctor will decide when the patient should stop and resume treatment with Formetic.
During treatment with Formetic, the doctor will monitor the patient's kidney function at least once a year or more frequently if the patient is elderly and/or has impaired kidney function.
Special care should be taken when using the medicine in situations where kidney function may be impaired (e.g., when starting treatment with a blood pressure-lowering medicine or a non-steroidal anti-inflammatory medicine).
The patient should inform their doctor about any bacterial or viral infection (e.g., flu, respiratory or urinary tract infection).
The patient should continue their diet while taking Formetic, with regular carbohydrate intake during the day (starchy foods like rice, pasta, potatoes, fruits). Patients who are overweight should also follow a low-calorie diet under medical supervision.
Children and adolescents:
Before taking Formetic, the diagnosis of type 2 diabetes should be confirmed by a doctor.
No effect of metformin on growth and pubertal development has been observed during one-year clinical studies, but long-term data in children and adolescents are not available.
Due to the limited number of children aged 10-12 years participating in clinical trials, children of this age should be treated with metformin with caution.
Elderly patients
Since kidney function in elderly patients is often impaired, the doctor should adjust the dose of Formetic according to kidney function. Therefore, regular assessment of kidney function by the doctor is necessary in these patients.
Formetic and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
If the patient is to receive an iodine-containing contrast agent intravenously, for example, for an X-ray examination or computed tomography, they should stop taking Formetic before or at the latest at the time of administration. The doctor will decide when the patient should stop and resume treatment with Formetic.
During treatment with Formetic, starting or stopping other medicines may have an adverse effect on blood glucose control.
The patient should inform their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. The patient may need more frequent blood glucose monitoring and kidney function tests or dose adjustments of Formetic by the doctor. It is especially important to inform about the following medicines:
- corticosteroids (e.g., prednisolone)
- diuretics,
- medicines used to treat pain and inflammation (NSAIDs and COX-2 inhibitors, such as ibuprofen and celecoxib),
- certain medicines used to treat high blood pressure (ACE inhibitors and angiotensin II receptor antagonists).
- medicines used to treat asthma (beta-sympathomimetics, e.g., salbutamol).
Taking Formetic with alcohol
The patient should avoid consuming excessive amounts of alcohol while taking Formetic, as this may increase the risk of lactic acidosis (see "Warnings and precautions").
Pregnancy, breastfeeding, and fertility
Women with diabetes who are pregnant or plan to become pregnant should not take Formetic. To maintain blood glucose levels as close to normal as possible, insulin should be used. The patient should inform their doctor about pregnancy or planned pregnancy, as the doctor should use insulin in such cases.
Formetic should not be taken during breastfeeding.
During pregnancy and breastfeeding, or if pregnancy is suspected or planned, the patient should consult their doctor or pharmacist before taking this medicine.
Driving and using machines
Taking metformin as the only medicine (monotherapy) does not cause significant hypoglycemia (low blood glucose levels), and therefore Formetic does not affect the ability to drive or use machines.
However, when metformin is used in combination with sulfonylurea derivatives, insulin, or other anti-diabetic medicines, the patient's ability to drive or use machines or perform work requiring concentration may be impaired due to hypoglycemia (with symptoms such as sweating, fainting, dizziness, or weakness).
3. How to take Formetic
This medicine should always be taken exactly as prescribed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
Treatment of type 2 diabetes
The dose of Formetic should be determined by the doctor based on blood glucose levels.
Formetic is available in different doses. To individualize the dose, tablets containing 500 mg or 1000 mg of metformin hydrochloride can be used. To facilitate swallowing, the 850 mg tablet can be divided.
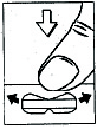
How to break the tablet:
If necessary, the coated tablet can be divided into two equal parts.
The tablet should be placed on a hard surface with the smaller dividing line facing down. Then, with the index finger, press the deeper dividing line.
The tablet will break into two parts.
If the doctor does not prescribe otherwise, the medicine is usually taken as described below.
Adults
The recommended initial dose is 1 coated tablet of Formetic (850 mg) 2 to 3 times a day (corresponding to 1700 mg to 2550 mg of metformin hydrochloride), taken during or after meals.
The maximum dose is 3 coated tablets of 850 mg per day (corresponding to 2550 mg of metformin hydrochloride), taken in divided doses.
If the patient has impaired kidney function, the doctor may prescribe a lower dose.
Children over 10 years old and adolescents:
Formetic used as the only anti-diabetic medicine or in combination with insulin:
The recommended initial dose is 1 coated tablet of Formetic (850 mg) per day (corresponding to 850 mg of metformin hydrochloride), taken during or after meals.
If necessary, the doctor may gradually increase the dose of Formetic up to a maximum dose of 2 coated tablets of 850 mg per day (corresponding to 1700 mg of metformin hydrochloride), taken in divided doses.
The coated tablets should be taken during or after meals, with a sufficient amount of fluid, and should not be chewed. If 2 or more coated tablets are taken per day, they should be taken at different times of the day, e.g., 1 coated tablet during or after breakfast and 1 coated tablet during or after lunch.
Pre-diabetic state
Usually, the initial dose is 500 mg (1 coated tablet of Formetic 500 mg or ½ coated tablet of Formetic 1000 mg) per day. Depending on the clinical response, the dose can be increased up to 1700 mg per day, given in divided doses.
If the patient feels that the effect of Formetic is too strong or too weak, they should consult their doctor.
Taking a higher dose of Formetic than recommended
In case of taking a higher dose of Formetic than recommended, the patient should immediately inform their doctor.
Overdose of Formetic does not cause hypoglycemia (significant decrease in blood glucose levels), but increases the risk of lactic acidosis.
Symptoms of impending lactic acidosis may be similar to metformin's gastrointestinal side effects: nausea, vomiting, diarrhea, abdominal pain with muscle cramps and weakness. In severe cases, muscle cramps, rapid breathing, decreased body temperature, acute kidney failure, and impaired consciousness or coma may also occur. These symptoms may develop within a few hours and require immediate hospital treatment.
Missing a dose of Formetic
In case of missing a dose of Formetic, the patient should take it at the time of the next scheduled dose and then continue with the prescribed dosing schedule. The patient should not take a double dose of the medicine to make up for the missed dose.
Stopping treatment with Formetic
In case of stopping treatment with Formetic, the patient should remember that poorly controlled, elevated blood glucose levels can cause damage to the eyes, kidneys, and blood vessels over a longer period.
In case of any further doubts about taking this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Formetic can cause side effects, although not everybody gets them.
Side effects are ranked according to the following frequency:
| Very common: occurring in more than 1 in 10 treated patients | Common: occurring in less than 1 in 10 treated patients and more than 1 in 100 treated patients |
| Uncommon: occurring in less than 1 in 100 treated patients and more than 1 in 1000 treated patients | Rare: occurring in less than 1 in 1000 treated patients and more than 1 in 10,000 treated patients |
| Very rare: occurring in less than 1 in 10,000 treated patients, frequency not known (cannot be estimated from the available data) | |
Formetic may very rarely cause (may occur in up to 1 in 10,000 patients) a very serious side effect called lactic acidosis (see "Warnings and precautions"). If this occurs, the patient should stop takingFormetic and immediately contact their doctor or the nearest hospital, as lactic acidosis can lead to coma.
Very common:
Nausea, vomiting, diarrhea, abdominal pain, and loss of appetite. These symptoms occur more frequently at the beginning of treatment and usually disappear on their own. To avoid gastrointestinal side effects, it is recommended to take Formetic in 2 or 3 divided doses during or immediately after meals. If these disorders persist for a longer period, the patient should stop taking Formetic and consult their doctor.
Common:
Taste disturbances.
Very rare:
Severe metabolic disorders associated with lactic acidosis (lactic acidosis). Symptoms of such disorders may include vomiting and abdominal pain, rapid breathing, muscle cramps, severe fatigue, decreased body temperature, acute kidney failure, or impaired consciousness (see "Warnings and precautions"). Lactic acidosis requires immediate hospital treatment. If the patient suspects that lactic acidosis has occurred (lactic acidosis), they should immediately consult their doctor and stop taking Formetic.
Skin reactions, such as rash, itching, and urticaria.
Abnormal liver function test results or hepatitis, with possible jaundice (yellowing of the skin and eyes), which disappears after stopping Formetic.
Decreased absorption of vitamin B12, leading to anemia, glossitis, paresthesia, and numbness in the limbs.
Children and adolescents
Limited information on side effects observed in children aged 10-16 years and adolescents suggests that they have a similar nature and frequency as in adults.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Formetic
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the blister or carton after "EXP". The expiry date refers to the last day of the month.
The inscription on the packaging after "EXP" means the expiry date, and after "Lot/LOT" means the batch number.
Storage conditions
There are no special precautions for storage.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Formetic contains
- The active substance of Formetic is metformin hydrochloride. Each coated tablet contains 850 mg of metformin hydrochloride, corresponding to 662.8 mg of metformin.
- The other ingredients of the medicine are: hypromellose (15,000 mPas), povidone (K 25), magnesium stearate, hypromellose (5 mPas), macrogol 6000, titanium dioxide (E171).
What Formetic looks like and contents of the pack
Formetic 850 mg is a white, elongated coated tablet with a dividing line on one side and a deep dividing line on the other.
The tablet can be divided into two equal parts.
Formetic is available in packs of 30, 60, 90, 120, 180 coated tablets.
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański, Poland
phone: +48 22 364 61 01
Manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański, Poland
Dragenopharm Apotheker Püschl GmbH
Göllstraße 1, 84529 Tittmoning, Germany
Date of last revision of the leaflet:March 2025
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterDragenopharm Apotheker Pueschl GmbH & Co. KG Zakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Formetic
Alternatives to Formetic in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Formetic in Hiszpania
Alternative to Formetic in Ukraina
Online doctors for Formetic
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Formetic – subject to medical assessment and local rules.










