How to use Fastum
LEAFLET INCLUDED IN THE PACKAGING: INFORMATION FOR THE PATIENT
Warning! Keep the leaflet, the information on the immediate packaging is in a foreign language!
Fastum(Fastum Gel)
25 mg/g, gel
Ketoprofen
Fastum and Fastum Gel are different trade names for the same drug.
You should carefully read the contents of the leaflet before using the medicine because it contains important information for the patient.
- You should keep this leaflet so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their illness symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Fastum and what is it used for.
- 2. Important information before using Fastum.
- 3. How to use Fastum.
- 4. Possible side effects.
- 5. How to store Fastum.
- 6. Contents of the packaging and other information.
1. What is Fastum and what is it used for
Fastum is a non-steroidal anti-inflammatory drug for topical use.
Ketoprofen, the active substance of Fastum, when applied topically, is absorbed through the skin into inflamed areas of joints, tendons, ligaments, and muscles. It does not accumulate in the body. The gel, thanks to its water-alcohol base, also has a surface cooling and soothing effect. The appropriate base ensures proper release of ketoprofen and minimal risk of systemic side effects. The drug is well tolerated even in the case of sensitive skin.
Indications for use
- Local treatment of muscle and joint pains, e.g., caused by injuries (injuries caused by playing sports, joint injuries with ligament sprains without dislocation, tendon and muscle damage caused by excessive exertion),
- Local treatment of low back pain in the course of discopathy.
2. Important information before using Fastum
When not to use Fastum
Do not use the medicine if you have a history of hypersensitivity to ketoprofen or any of the other ingredients of Fastum, tiaprofenic acid, acetylsalicylic acid, or other non-steroidal anti-inflammatory drugs, fenofibrate, UV filters, or perfumes.
Ketoprofen should not be used in patients who experience "aspirin-induced asthma" attacks, rash, or rhinitis after using acetylsalicylic acid and its derivatives.
The medicine is contraindicated in people with a history of aspirin-induced asthma or other hypersensitivity reactions.
Fastum should not be used on open wounds or in people with eczema or oozing skin lesions of unknown origin.
Stop using the medicine immediately if skin reactions occur, including skin reactions after concurrent use of products containing octocrylene (octocrylene is one of the excipients used in various cosmetics and hygiene products such as shampoo, after-shave products, shower gels and bath products, skin creams, lip balms, anti-wrinkle creams, makeup removers, hair lacquers, to delay decomposition under the influence of light).
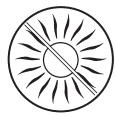
Do not expose the treated skin area to sunlight (even on cloudy days) or UV rays in a solarium during treatment and for 2 weeks after stopping the use of the medicine.
Ketoprofen is not recommended for children under 15 years of age due to the lack of controlled clinical trials regarding its efficacy and safety. Do not use in women from the sixth month of pregnancy.
Warnings and precautions
Exposure to sunlight (even on cloudy days) or UVA rays on skin areas where Fastum has been applied may cause severe skin reactions (photosensitivity). Therefore, it is necessary:
- to protect the treated skin areas by wearing clothing during treatment and for 2 weeks after stopping the use of the medicine to avoid the risk of photosensitivity
- to wash your hands thoroughly after each application of Fastum. Prolonged use of topical medicines may cause allergic reactions or local irritation in some patients. If redness, rash, itching, or nausea occur, stop using the medicine and consult a doctor. The medicine should not be used on open wounds or cuts, and if skin changes occur at the site of application, the medicine should be discontinued. Protect your eyes and mucous membranes from contact with the medicine, do not use under occlusive dressings. The medicine should not be used orally or on large areas of the body. Local administration of large amounts of the medicine may cause systemic effects, including hypersensitivity reactions and asthma. The medicine should be used with caution in patients with circulatory failure, severe kidney or liver dysfunction, as individual cases of systemic side effects have been reported. In patients with a history of epilepsy, caution should be exercised when using the medicinal product due to the presence of a terpene derivative (lavender aroma) in the product composition. After applying the gel, wash your hands unless they are the area being treated. During prolonged use, it is recommended to wear protective gloves. Treatment should be stopped immediately if any skin reactions occur after using Fastum. The medicine contains alcohol, do not use near fire. In patients with asthma and chronic rhinitis, chronic sinusitis, and (or) nasal polyps, the risk of an allergic reaction to aspirin and (or) NSAIDs is higher than in the rest of the population.
Fastum and other medicines
No interactions between Fastum and other medicines have been reported. However, caution should be exercised in patients taking oral anticoagulant medicines at the same time.
Pregnancy
Before using any medicine, consult a doctor.
Using non-steroidal anti-inflammatory drugs (NSAIDs) during the third trimester of pregnancy may have a harmful effect on the fetus's heart and lungs. Non-steroidal anti-inflammatory drugs may cause delayed labor. The use of the medicinal product should be avoided during the first and second trimester of pregnancy. Do not use in women from the third trimester of pregnancy.
Breast-feeding
Before using any medicine, consult a doctor.
Ketoprofen passes into breast milk in small amounts. The use of the medicine is not recommended for breastfeeding women.
Driving and using machines
The effect of the medicinal product on the ability to drive and use machines has not been described.
Fastum contains orange aroma and lavender aroma.
This medicine contains aromas with the following composition: citral, citronellol, coumarin, farnesol, geraniol, d-limonene, and linalool, which may cause allergic reactions.
3. How to use Fastum
Fastum is used 1 to 2 times a day, apply a thin layer of gel (a strip of gel 3 cm to 5 cm long) to the skin in the painful area and gently massage to facilitate absorption. If after 7 days of using the medicine the symptoms do not disappear, worsen, or new symptoms appear, consult a doctor.
Fastum should not be used in children under 15 years of age.
Using a higher dose of Fastum than recommended
No cases of overdose or poisoning with Fastum have been reported.
In the event of accidental ingestion of the gel, the following may occur: drowsiness, nausea, vomiting. Ingestion of large doses may cause respiratory depression, coma, convulsions, gastrointestinal bleeding, increased or decreased blood pressure, acute kidney failure. The doctor will take appropriate measures and initiate symptomatic treatment usually used in the treatment of NSAID poisoning. If it has been less than 1 hour since the overdose, gastric lavage should be performed and symptomatic treatment should be administered.
4. Possible side effects
Like all medicines, Fastum can cause side effects, although not everybody gets them.
The following frequencies are usually the basis for assessing side effects:
Very common: more than 1 in 10 treated patients;
Common: less than 1 in 10 but more than 1 in 100 treated patients;
Uncommon: less than 1 in 100 but more than 1 in 1,000 treated patients;
Rare: less than 1 in 1,000 but more than 1 in 10,000 treated patients;
Very rare: less than 1 in 10,000 treated patients, not known (cannot be estimated from the available data).
There have been reports of local skin reactions that may spread beyond the application site. Rarely, severe reactions such as bullous or papular rashes have occurred, which may spread or become generalized.
Other systemic side effects (e.g., related to the gastrointestinal tract or kidneys) caused by non-steroidal anti-inflammatory drugs result from the absorption of the active substance through the skin and are therefore dependent on the amount of gel applied, the treated skin surface, the degree of absorption into the tissue, the duration of therapy, and the possible use of an occlusive dressing (hypersensitivity, gastrointestinal disorders, and kidney function disorders).
Since the marketing authorization of the medicinal product, the following side effects have been observed, which are listed below, grouped by organ system and frequency of occurrence as: very common (greater than or equal to 10%), common (between 1% and 10%), uncommon (between 0.1% and 1%), rare (between 0.01% and 0.1%), or very rare (less than 0.01%) including individual cases.
Immune system disorders
Very rare:
Gastrointestinal disorders
Anaphylactic reaction, hypersensitivity reactions
Very rare:
Ulcerative disease of the stomach and (or) duodenum, gastrointestinal bleeding, diarrhea
Skin and subcutaneous tissue disorders
Uncommon:
Redness, itching, rash, burning
Rare:
Photosensitivity, bullous rash, urticaria
Very rare:
Contact dermatitis, angioedema
Kidney and urinary tract disorders
Very rare:
Kidney failure or worsening of kidney function disorders
Patients of advanced age are particularly at risk of side effects after administration of non-steroidal anti-inflammatory drugs.
If any of the side effects worsen or any side effects not listed in this leaflet occur, you should inform your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, you should tell your doctor or pharmacist, or nurse.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al.
Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or parallel importer.
Reporting side effects will allow for more information to be collected on the safety of the medicine.
5. How to store Fastum
There are no special requirements for storage.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the given month. Shelf life after first opening of the packaging
: 6 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Fastum contains
- The active substance of the medicine is: Ketoprofen(ketoprofen).
- Other ingredients of the medicine are: carbomer, ethanol 96%, triethanolamine, orange aroma (contains citral, citronellol, farnesol, geraniol, d-limonene, and linalool), lavender aroma (contains coumarin, geraniol, d-limonene, and linalool), purified water.
What Fastum looks like and what the packaging contains
A tube containing 20 g, 30 g, 50 g, or 100 g of gel.
To obtain more detailed information, please contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Romania, the country of export:
- A. Menarini Industrie Farmaceutiche Riunite S.r.l. Via Sette Santi 3 50131 Florence Italy
Manufacturer
- A. Menarini Manufacturing Logistics and Services S.r.l. Via Sette Santi 3 50131 Florence Italy
Parallel importer:
Medezin Sp. z o.o.
Zbąszyńska 3 Street
91-342 Łódź
Repackaged by:
Medezin Sp. z o.o.
Zbąszyńska 3 Street
91-342 Łódź
CEFEA Sp. z o.o. Sp. komandytowa
Działkowa 56 Street
02-234 Warsaw
Pharma Innovations Sp. z o.o.
Jagiellońska 76 Street
03-301 Warsaw
SHIRAZ PRODUCTIONS Sp. z o.o.
Tymiankowa 24/28 Street
95-054 Ksawerów
CANPOLAND SPÓŁKA AKCYJNA
Beskidzka 190 Street
91-610 Łódź
Marketing authorization number in Romania, the country of export:
8884/2016/01
8884/2016/02
8884/2016/04
8884/2016/03
Parallel import authorization number: 320/22 Date of approval of the leaflet: 25.08.2022
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)A. Menarini Industrie Farmaceutiche Riunite S.r.l.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FastumDosage form: Gel, 25 mg/gActive substance: ketoprofenManufacturer: A.Menarini Manufacturing Logistics and Services s.r.l.Prescription requiredDosage form: Gel, 25 mg/gActive substance: ketoprofenPrescription requiredDosage form: Gel, 25 mg/gActive substance: ketoprofenPrescription required
Alternatives to Fastum in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fastum in Ukraine
Alternative to Fastum in Spain
Online doctors for Fastum
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fastum – subject to medical assessment and local rules.