
Eziclen

Ask a doctor about a prescription for Eziclen

How to use Eziclen
Leaflet accompanying the packaging: information for the user
Eziclen, concentrate for solution for oral use
Sodium sulfate anhydrous + Magnesium sulfate heptahydrate + Potassium sulfate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Eziclen and what is it used for
- 2. Important information before using Eziclen
- 3. How to use Eziclen
- 4. Possible side effects
- 5. How to store Eziclen
- 6. Contents of the packaging and other information
1. What is Eziclen and what is it used for
Eziclen contains three different active substances: sodium sulfate, magnesium sulfate, and potassium sulfate.
Eziclen is used in adults to cleanse the intestine before a medical examination or surgical procedure on the intestine.
Eziclen is not a laxative.
Eziclen must be diluted with water before use, as described in the administration method (see section 3).
2. Important information before using Eziclen
When not to use Eziclen:
- If the patient is allergic to sodium sulfate, magnesium sulfate, or potassium sulfate, or any of the other ingredients of this medicine (listed in section 6).
- If the patient has severe heart problems (congestive heart failure).
- If the patient has severe dehydration.
- If the patient has acute phases of active inflammatory bowel disease (including Crohn's disease and ulcerative colitis).
- If the patient has any of the following stomach or intestinal disorders:
- known or suspected intestinal obstruction or narrowing
- toxic megacolon or toxic colonic dilation
- diagnosed or suspected perforation of the intestinal wall
- intestinal paralysis
- problems with stomach emptying (e.g., gastric paralysis, gastric stasis)
- acute abdominal conditions requiring surgery, such as acute appendicitis
- nausea and vomiting.
- If the patient has ascites.
- If the patient has severe kidney problems (severe renal failure).
In case of doubts, consult the doctor before using Eziclen.
Warnings and precautions
Before starting Eziclen, consult a doctor if:
- The patient is dehydrated (symptoms may include dry mouth, thirst, headache, dizziness, urinating less often than usual, extreme fatigue, palpitations, and confusion)
- The patient has ever had abnormal sodium or potassium levels in the blood
- The patient has heart disease. Eziclen may affect heart rhythm due to changes in blood salt levels. Monitoring of the patient during treatment may be necessary.
- The patient has kidney disease (renal failure)
- Dehydration may cause kidney problems (renal failure), which are reversible with proper treatment.
- The patient has liver disease.
- The patient has disorders related to "uric acid" (gout or other conditions).
- The patient has swallowing disorders.
- The patient has "reflux" when stomach acid flows back into the esophagus.
- The patient is physically weakened or in poor health.
- The patient has weakened intestinal function (impaired peristalsis).
- The patient has a history of health conditions or surgical procedures related to the digestive tract, which may predispose to impaired peristalsis of the digestive tract.
If the patient is weakened or elderly (65 years or older), has severe kidney, liver, or heart disease, or is at risk of changes in salt levels in the body (electrolyte disturbances), the doctor may recommend special monitoring of the patient before and after the examination or procedure. The patient should pay special attention to the recommendations listed in this part of the leaflet as well as in the "Other medicines and Eziclen" and "How to use Eziclen" sections.
If the patient experiences severe vomiting or symptoms of dehydration (e.g., dry mouth, thirst) after taking this medicine, they should inform their doctor, who will recommend the use of hydrating measures.
If severe or persistent abdominal pain and/or rectal bleeding occur after taking Eziclen, the patient should consult a doctor. During the use of Eziclen, there have been rare cases of colitis (colonic inflammation).
If any of the above points apply to the patient (or in case of uncertainty), they should consult their doctor before using Eziclen.
After taking the medicine, the patient will experience frequent loose stools. This is normal and indicates the effective action of the medicine. The patient should stay near a toilet until the effect of the medicine wears off.
The patient should strictly follow the recommendations and drink as much water or clear fluids as needed to avoid dehydration.
Children and adolescents
Eziclen is not intended for use in patients under 18 years of age. The safety and efficacy of this medicine in this patient group have not been established.
Other medicines and Eziclen
Tell the doctor or pharmacist about all medicines currently being used or recently used, as well as any medicines the patient plans to take .This includes over-the-counter medicines and herbal products.
If the patient is taking any other medicines, they should take them one to three hours before taking Eziclen or at least one hour after completing the bowel cleansing process.
This is because the diarrhea caused by Eziclen may flush out other medicines from the body, making them less effective.
Special precautions should be taken when using:
- medicines that may affect fluid or salt levels in the blood (such as diuretics, calcium channel blockers, or lithium salts) or medicines that affect heart rhythm.
- oral medicines taken regularly, such as oral contraceptives, antiepileptics, antidiabetics, antibiotics, levothyroxine (a hormone used to treat hypothyroidism), or digoxin (used in heart conditions), as Eziclen may delay or completely inhibit the absorption of these medicines, reducing or eliminating their effect.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
During breastfeeding, the patient should not breastfeed their child for 48 hours after taking the second dose of Eziclen.
Driving and using machines
Eziclen is unlikely to affect the ability to drive or use tools or machines.
Eziclen contains sodium and potassium
If the patient is on a controlled salt diet (sodium or potassium), they should note that each bottle of Eziclen contains 5.684 g (247.1 mmol) of sodium and 1.405 g (35.9 mmol) of potassium.
3. How to use Eziclen
This medicine should always be used exactly as described in this patient leaflet or as directed by the doctor. In case of doubts, consult a doctor or pharmacist.
Using the medicine
The medicine should be taken orally.
The day before the examination or procedure, the patient can have a light breakfast. After breakfast, they should only drink clear fluids for lunch, dinner, and any other meals until the examination or procedure is performed. The patient should not drink red or purple beverages, milk, or alcoholic beverages.
Eziclen is supplied in 2 bottles, packed in a box with a measuring cup for dilution and administration of the medicine. All these elements are necessary for the administration of the medicine.
Do notdrink the contents of both bottles at the same time.
Do notdrink the contents of the bottles before diluting them.
Do notforget to drink additional water or clear fluids.
The doctor will provide the patient with a form to record the start time of treatment and the amount of fluids taken during bowel preparation.
The patient should strictly follow the recommendations and drink as much water or clear fluids as needed to avoid dehydration.
“Clear fluids” include water, tea, or coffee (without milk or non-dairy creamer), carbonated or non-carbonated drinks, strained fruit juices without pulp (of any color other than red or purple), clear soup, or soup strained to remove all solid elements.
The patient should not drink alcoholic beverages.
How and when to use this medicine
Eziclen can be taken in a "Two-Day" or "One-Day" regimen. The doctor will decide which regimen and at what time the medicine should be taken.
- In the case of an examination or procedure without general anesthesia, the patient should stop drinking the fluid at least one hour before the examination or procedure.
- In the case of an examination or procedure with general anesthesia, the patient should stop drinking the fluid usually at least two hours before the examination or procedure, following the anesthesiologist's instructions.
- Two-Day regimenThe dosing is divided into the evening of the day before the examination or procedure and the morning of the day of the examination or procedure. The evening before the examination or procedure:
- take the first part of the regimen (first bottle) in the early evening (i.e., no later than 6:00 PM). On the day of the examination or procedure:
- take the second part of the regimen (second bottle) in the early morning, 10 to 12 hours after starting the first part of the regimen (first bottle).
- One-Day regimenThe dosing starts and ends in the evening of the day before the examination or procedure. The evening before the examination or procedure:
- take the first part of the regimen (first bottle) in the early evening (i.e., no later than 6:00 PM)
- take the second part of the regimen (second bottle) about 2 hours after starting the first part of the regimen (first bottle).
Regardless of the regimen chosen by the doctor, the following steps should be performed for both the first and second parts of the regimen:
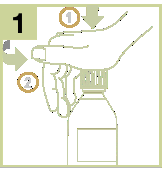
- 1) Open the child-resistant bottle by pressing the cap and twisting it in the opposite direction of the clockwise direction.
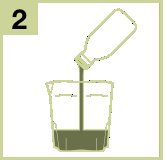
- 2) Pour the contents of one Eziclen bottle into the measuring cup.
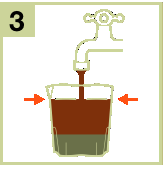
- 3) Add water to the medicine up to the mark on the measuring cup.
- 4) Drink the entire contents of the measuring cup without rushing (within 30 minutes to 1 hour).
- 5) IMPORTANT INFORMATION: Drink two (2) additional measuring cups of water or clear fluid. Each time, fill the measuring cup with water or clear fluid up to the mark.
- 6) Drink the entire contents of each measuring cup without rushing (within 30 minutes).
Performing the steps listed in points 1 to 6 takes about 2 hours and should be repeated for the second part of the regimen.
Regardless of the regimen used, the patient should stop drinking the fluid:
- at least one hour before the examination or procedure, in the case of an examination or procedure without general anesthesia,
- usually at least two hours before the examination or procedure, in the case of an examination or procedure with general anesthesia, following the anesthesiologist's instructions.
Using more than the recommended dose of Eziclen
If the patient thinks they have taken too much Eziclen or did not dilute the medicine as recommended, or did not drink enough additional water, they should inform their doctor and drink enough water or clear fluids to avoid dehydration.
Missing a dose of Eziclen
If a dose is missed, the patient should contact their doctor as soon as possible, as this may mean that the medicine will not work as expected.
In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
If rectal bleeding occurs during or after bowel preparation, the patient should contact their doctor.
Like all medicines, Eziclen can cause side effects, although not everybody gets them.
The following side effects may occur when using Eziclen:
If any of the following symptoms occur, the patient should stop using Eziclen and consult their doctor immediately:
Allergic reaction – symptoms may include skin rash or redness, itching, difficulty breathing, or swelling of the throat.
Other side effects include:
Very common (may affect more than 1 in 10 people)
General discomfort
Nausea or vomiting
Bloating or stomach pain.
Uncommon (may affect up to 1 in 100 people)
Chills
Dry mouth
Headache
Dizziness
Pain when urinating
Discomfort in the anal or rectal area
Changes in the levels of certain blood components. Examples include: increased activity of "aspartate aminotransferase", "creatine phosphokinase", "lactate dehydrogenase", increased levels of "phosphates", "bilirubin", or "uric acid", and decreased levels of "sodium", "potassium", or "calcium".
Frequency not known (frequency cannot be estimated from the available data):
Intestinal ulcers or disorders (ischemic colitis). The patient should immediately tell their doctor if they experience severe stomach pain or rectal bleeding.
Dehydration, changes in blood salt levels, fatigue, trembling, sweating, palpitations, loss of consciousness (seizures), disorientation, muscle cramps.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Eziclen
- The medicine should be stored out of sight and reach of children.
- Do not use this medicine after the expiry date stated on the carton and bottle after: Expiry Date (EXP). The expiry date refers to the last day of the month stated.
- There are no special storage temperature requirements for the medicine.
- Store in the original packaging to protect from light.
- After opening the bottle and/or diluting the contents with water, the solution should be used immediately.
- Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Eziclen contains
- The active substances are: sodium sulfate anhydrous, magnesium sulfate heptahydrate, and potassium sulfate. Each bottle of approximately 176 ml of concentrate contains 17.510 g of sodium sulfate anhydrous, 3.276 g of magnesium sulfate heptahydrate, and 3.130 g of potassium sulfate. The total electrolyte content is as follows:
Content in g
Content in mmol
1 bottle
2 bottles
1 bottle
2 bottles
Sodium*
5.684
11.367
247.1
494.42
Potassium
1.405
2.81
35.9
71.8
Magnesium
0.323
0.646
13.3
26.6
Sulfates
14.845
29.690
154.5
309.0
* derived from sodium sulfate (active substance) and sodium benzoate (excipient).
The other ingredients are sodium benzoate (E 211), anhydrous citric acid, sucralose, malic acid, purified water, and fruit cocktail flavor [a mixture of natural and synthetic flavors, propylene glycol (E 1520), ethanol, acetic acid, and benzoic acid (E 210)].
What Eziclen looks like and what the pack contains
- The medicine is a concentrate for solution for oral use, the concentrate is clear or slightly cloudy.
- It is supplied in 2 bottles of approximately 176 ml each, packed in a carton with a measuring cup, with a capacity of approximately half a liter, for dilution and administration of the medicine.
- Available pack sizes:
- 1 pack containing 2 bottles and 1 measuring cup
- 24 x 1 pack containing 2 bottles and 1 measuring cup
- 6 x 24 (=144) x 1 pack containing 2 bottles and 1 measuring cup
- 14 x 24 (=336) x 1 pack containing 2 bottles and 1 measuring cup
Not all pack sizes may be marketed.
Marketing authorization holder:
MAYOLY PHARMA FRANCE
3 Place Renault
92500 Rueil-Malmaison
France
Manufacturer:
MAYOLY INDUSTRIE
rue Ethé Virton
28100 Dreux
France
To obtain more detailed information about this medicine or to obtain a leaflet in a different format, the patient should contact the local representative of the marketing authorization holder:
MAYOLY POLSKA Sp. z o.o.
ul. Domaniewska 39B
02-672 Warsaw
phone: 22 166 26 26
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Belgium, France, Italy, United Kingdom (Northern Ireland)
Izinova
Czech Republic, Estonia, Greece, Netherlands, Lithuania, Luxembourg, Latvia, Germany, Portugal, Romania
Eziclen
Date of last revision of the leaflet:March 2025
- Country of registration
- Prescription requiredYes
- ImporterMAYOLY INDUSTRIE
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EziclenDosage form: Powder, 13.7 gActive substance: macrogol, combinationsPrescription not requiredDosage form: Powder, -Active substance: macrogol, combinationsManufacturer: Sigma Italia S.p.A.Prescription requiredDosage form: Tablets, 1500 mgActive substance: sodium phosphatePrescription required
Alternatives to Eziclen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Eziclen in Іспанія
Online doctors for Eziclen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Eziclen – subject to medical assessment and local rules.














